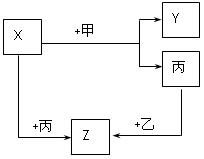
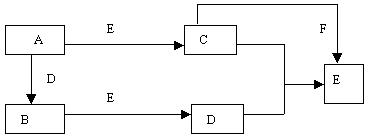
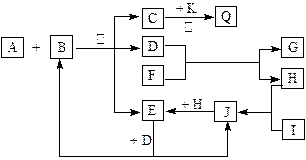��Ŀ����
�� 13�֣��±���Ԫ�����ڱ���һ���֣���ش��й����⣺(��Ԫ�ط���)
��1��aԪ����eԪ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ĽṹΪʽ��_____________��
��2��nԪ����fԪ�����ߺ˵����֮����_____________________��
��3���ϱ��У�ԭ�Ӱ뾶�����ǣ�ϡ��������⣩________________��
��4���Ʋ�j��d����⻯����ȶ���ǿ����ϵ____________���ѧʽ����
��5��c2a4��n�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ______________________________��
��6��д��c5a12������ͬ���칹��Ľṹ��ʽ_________________________��
| | IA | ��A | | ��A | ��A | VA | ��A | ��A | 0 | |
| 1 | a | | b | |||||||
| 2 | | | | | c | d | e | f | g | |
| 3 | h | | i | j | | | k | l | ||
| 4 | m | | | | | | n | | ||
��2��nԪ����fԪ�����ߺ˵����֮����_____________________��
��3���ϱ��У�ԭ�Ӱ뾶�����ǣ�ϡ��������⣩________________��
��4���Ʋ�j��d����⻯����ȶ���ǿ����ϵ____________���ѧʽ����
��5��c2a4��n�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ______________________________��
��6��д��c5a12������ͬ���칹��Ľṹ��ʽ_________________________��
��1��H��O��O��H ��2��26 ��3��K ��4��NH3 > SiH4
��5��CH2=CH2+Br2 ��CH2BrCH2Br
��6��CH3CH2CH2CH2CH3 CH3CH2CH(CH3)2 C(CH3)4 (3��)
��5��CH2=CH2+Br2 ��CH2BrCH2Br
��6��CH3CH2CH2CH2CH3 CH3CH2CH(CH3)2 C(CH3)4 (3��)
��1��aԪ����eԪ�طֱ���H��O������ԭ�Ӹ�����Ϊ1:1�Ļ�������H2O2���ṹʽΪH��O��O��H��
��2��nԪ����fԪ�طֱ���F��Br�����ߵĺ˵����֮����35��9��26��
��3��ͬ�����������ң�ԭ�Ӱ뾶����ͬ�������϶���ԭ�Ӱ뾶����������ԭ�Ӱ뾶������K��
��4��j��d�ֱ��ǹ��N��N�ķǽ�����ǿ�ڹ�ġ����ǽ�����Խǿ����Ӧ�⻯����ȶ���Խǿ�������⻯����ȶ�����NH3 > SiH4��
��5��c2a4��n�ĵ��ʷֱ�����ϩ�͵����壬���߷����ӳɷ�Ӧ������ʽΪCH2=CH2+Br2 ��CH2BrCH2Br��
��6��c5a12�����飬����3��ͬ���칹�壬�ֱ���CH3CH2CH2CH2CH3��CH3CH2CH(CH3)2��C(CH3)4��
��2��nԪ����fԪ�طֱ���F��Br�����ߵĺ˵����֮����35��9��26��
��3��ͬ�����������ң�ԭ�Ӱ뾶����ͬ�������϶���ԭ�Ӱ뾶����������ԭ�Ӱ뾶������K��
��4��j��d�ֱ��ǹ��N��N�ķǽ�����ǿ�ڹ�ġ����ǽ�����Խǿ����Ӧ�⻯����ȶ���Խǿ�������⻯����ȶ�����NH3 > SiH4��
��5��c2a4��n�ĵ��ʷֱ�����ϩ�͵����壬���߷����ӳɷ�Ӧ������ʽΪCH2=CH2+Br2 ��CH2BrCH2Br��
��6��c5a12�����飬����3��ͬ���칹�壬�ֱ���CH3CH2CH2CH2CH3��CH3CH2CH(CH3)2��C(CH3)4��

��ϰ��ϵ�д�
�����Ŀ