��Ŀ����
����״����4.48L��CO2ͨ��200mL��NaOH��Һǡ�ó�ַ�Ӧ����Һ�������ε�����Ϊ19.0g��
��1����Ӧ�����ɵ����ǣ�д��ѧʽ��
��2��200mLNaOH��Һ�����ʵ���Ũ��Ϊ
��3�������ɵ�19.0g������Һ�м���һ����ij���ʣ���ַ�Ӧ��ѹ���������õ�������21.2g Na2CO3���壮��
����ֻ�ܼ���0.05molij���ʣ������Ļ����������
����ֻ�ܼ���0.10molij���ʣ������Ļ����������
��1����Ӧ�����ɵ����ǣ�д��ѧʽ��
Na2CO3��NaHCO3
Na2CO3��NaHCO3
����2��200mLNaOH��Һ�����ʵ���Ũ��Ϊ
1.5mol/L
1.5mol/L
����3�������ɵ�19.0g������Һ�м���һ����ij���ʣ���ַ�Ӧ��ѹ���������õ�������21.2g Na2CO3���壮��
����ֻ�ܼ���0.05molij���ʣ������Ļ����������
Na2O��Na2O2
Na2O��Na2O2
������ֻ�ܼ���0.10molij���ʣ������Ļ����������
Na��NaOH
Na��NaOH
����������1��������̼���������Ʒ�����ӦCO2+2NaOH�TNa2CO3+H2O��CO2+NaOH�TNaHCO3����ֻ����̼���ƣ����ݶ�����̼���������̼������������ֻ����̼�����ƣ����ݶ�����̼���������̼�������������ݼ�������̼���ơ�̼�����Ƶ��������ж϶�����̼�������������ɵ��ε���ɣ������ε���ɿ�֪��ʹ�����صľ��巴Ӧ����Ϸ���ʽ���ݲ��������㣻
��2������Cԭ���غ��֪��19.0 g�ε����ʵ������ڶ�����̼�����ʵ���������Na2CO3��NaHCO3��ƽ��Ħ������������ʮ�ֽ��淨����Na2CO3��NaHCO3�����ʵ���֮�ȣ���������������ʵ������ٸ�����Ԫ���غ����NaOH���ʵ�����
��3��21.2g Na2CO3��������ʵ���Ϊ0.2mol������19.0g�ε���ɣ����Cԭ�ӡ�Naԭ���غ�����жϣ�
��2������Cԭ���غ��֪��19.0 g�ε����ʵ������ڶ�����̼�����ʵ���������Na2CO3��NaHCO3��ƽ��Ħ������������ʮ�ֽ��淨����Na2CO3��NaHCO3�����ʵ���֮�ȣ���������������ʵ������ٸ�����Ԫ���غ����NaOH���ʵ�����
��3��21.2g Na2CO3��������ʵ���Ϊ0.2mol������19.0g�ε���ɣ����Cԭ�ӡ�Naԭ���غ�����жϣ�
����⣺��1��������̼���������Ʒ�Ӧ����ֻ����̼���ƣ����ݷ���ʽ��϶�����̼���������̼������������
CO2+2NaOH�TNa2CO3+H2O
22.4L 106g
4.48L m
����m=21.2 g��
��ֻ����̼�����ƣ����ݶ�����̼���������̼����������
CO2+NaOH�TNaHCO3
22.4L 84g
4.48L n
����n=16.8 g��
����16.8 g��19.0g��21.2 g����19.0 g��ӦΪNa2CO3��NaHCO3�Ļ���
�ʴ�Ϊ��Na2CO3��NaHCO3��
��2������Cԭ���غ��֪��19.0 g�ε����ʵ������ڶ�����̼�����ʵ���Ϊ
=0.2mol������Na2CO3��NaHCO3��ƽ��Ħ������Ϊ
=95g/mol������ʮ�ֽ��淨����Na2CO3��NaHCO3�ĸ������ʵ�����
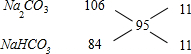
����Na2CO3��NaHCO3�����ʵ���֮��Ϊ11��11=1��1������Na2CO3Ϊ0.1 mol��NaHCO3Ϊ0.1 mol��
������Ԫ���غ��֪NaOH���ʵ���Ϊ0.1mol��2+0.1mol=0.3mol
����200mLNaOH��Һ�����ʵ���Ũ��Ϊ
=1.5mol/L��
�ʴ�Ϊ��1.5mol/L��
��3��19.0 g��ΪNa2CO3Ϊ0.1 mol��NaHCO3Ϊ0.1 mol��Ҫ�õ�������21.2g Na2CO3���壬��Ҫ��0.1 mol NaHCO3��Ϊ0.1 mol Na2CO3��
��ij�����ʡ�������3��������a���Լ��Ի������ɼb�������NaԪ�أ�c�����ܴ����������ʣ�
���и���Naԭ���غ㣬0.05 mol����������0.1 mol Naԭ�ӣ��ʷ���������ֻ��Na2O��Na2O2��
�ʴ�Ϊ��Na2O��Na2O2��
���и���Naԭ���غ�0.1 mol����������0.1 mol Na���ʷ��������Ŀ���Na��NaOH�ȣ�
�ʴ�Ϊ��Na��NaOH��
CO2+2NaOH�TNa2CO3+H2O
22.4L 106g
4.48L m
����m=21.2 g��
��ֻ����̼�����ƣ����ݶ�����̼���������̼����������
CO2+NaOH�TNaHCO3
22.4L 84g
4.48L n
����n=16.8 g��
����16.8 g��19.0g��21.2 g����19.0 g��ӦΪNa2CO3��NaHCO3�Ļ���
�ʴ�Ϊ��Na2CO3��NaHCO3��
��2������Cԭ���غ��֪��19.0 g�ε����ʵ������ڶ�����̼�����ʵ���Ϊ
| 4.48L |
| 22.4L/mol |
| 19g |
| 0.2mol |
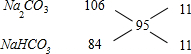
����Na2CO3��NaHCO3�����ʵ���֮��Ϊ11��11=1��1������Na2CO3Ϊ0.1 mol��NaHCO3Ϊ0.1 mol��
������Ԫ���غ��֪NaOH���ʵ���Ϊ0.1mol��2+0.1mol=0.3mol
����200mLNaOH��Һ�����ʵ���Ũ��Ϊ
| 0.3mol |
| 0.2L |
�ʴ�Ϊ��1.5mol/L��
��3��19.0 g��ΪNa2CO3Ϊ0.1 mol��NaHCO3Ϊ0.1 mol��Ҫ�õ�������21.2g Na2CO3���壬��Ҫ��0.1 mol NaHCO3��Ϊ0.1 mol Na2CO3��
��ij�����ʡ�������3��������a���Լ��Ի������ɼb�������NaԪ�أ�c�����ܴ����������ʣ�
���и���Naԭ���غ㣬0.05 mol����������0.1 mol Naԭ�ӣ��ʷ���������ֻ��Na2O��Na2O2��
�ʴ�Ϊ��Na2O��Na2O2��
���и���Naԭ���غ�0.1 mol����������0.1 mol Na���ʷ��������Ŀ���Na��NaOH�ȣ�
�ʴ�Ϊ��Na��NaOH��
���������������̼���������Ʒ�Ӧ�ļ��㣬�ѶȽϴؼ��������ü���ȷ��19.0g���εijɷ֣�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ