��Ŀ����
���ⶨ�������Ȼ��Ƶ�С�մ��̬��Ʒ��NaHCO3�����������ɲ����������ַ�����
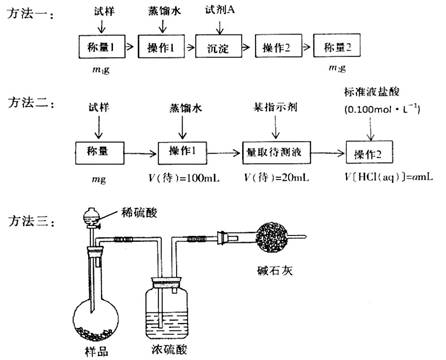
�����ģ���ʹ�û�ѧ�Լ���ʹ��ʵ���ҳ���������
��Ҫ��ش��������⣺
��1������һ�ǽ�HCO3-����ת��Ϊ���������أ����Լ�AΪ______________���ѧʽ����Һ������2����_______________________��
��2������������1��Ҫ�õ��IJ����������ձ���������������Ҫ___________________������2��������__________������Ʒ��NaHCO3����������Ϊ_________���ú�m��a�ı���ʽ��ʾ����
��3�����ݷ����������õ�ʵ��װ�ã����˳������������⣬����ⶨ��ʵ��������_______________����ϸ������ʵ��װ�ã��ɴ˲�õ����ݼ������ʵ�����п���ƫ��Ҳ�п���ƫ�ͣ�ƫ�ߵ�ԭ�������______________________________��ƫ�͵�ԭ�������____________________________��
��4�������ĵ�ʵ��ԭ����________________���û�ѧ����ʽ��ʾ����
��9�֣���1��Ba(OH)2[��Ca(OH)2]�����ˡ�ϴ�ӡ�����д����÷֣�
��2��100ml����ƿ����ͷ�ιܣ��к͵ζ��� ����
���� ��
��
��3��m(CO2)����ʵ��ǰ�����ܵ��������������еĶ�����̼��ˮ�����Ƚ������ܣ�װ���еĶ�����̼û����ȫ��������
��4��2NaHCO3 Na2CO3��H2O��CO2����ÿ��1�֣�
Na2CO3��H2O��CO2����ÿ��1�֣�
��������
�����������1��Ҫ��HCO3-����ת��Ϊ���������أ�����Ҫ����ǿ�������������������ƣ���ѧʽ�ֱ�ΪBa(OH)2��Ca(OH)2�����������ʴ���Һ�з�����IJ����ǹ��ˣ����õij�������Ҫ����ϴ�Ӳ��������ܳ�����
��2��Ҫ����100ml����Һ�������1��Ҫ�õ��IJ��������������ձ����������⣬����Ҫ100ml����ƿ���Լ�����ʱ�Ľ�ͷ�ιܡ�������ζ�̼��������Һ�����Բ���2���������к͵ζ���20ml����Һ������������ʵ�����a��10��4mol������ݷ���ʽNaHCO3��HCl��NaCl��H2O��CO2����֪��ԭ��Ʒ��̼�����Ƶ����ʵ�����a��10��4mol��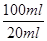 ��5a��10��4mol����������5a��10��4mol��84g/mol��0.042ag�����Ը���Ʒ��NaHCO3����������Ϊ
��5a��10��4mol����������5a��10��4mol��84g/mol��0.042ag�����Ը���Ʒ��NaHCO3����������Ϊ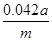 ��
��
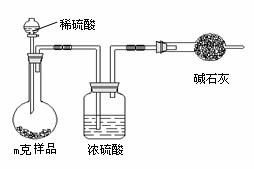
��3��̼�����������ᷴӦ����CO2����ͨ������CO2���������������̼�����Ƶĺ��������Ը��ݷ����������õ�ʵ��װ�ã����˳������������⣬����ⶨ��ʵ��������m(CO2)��ʵ��ǰ�����ܵ����������ڸ������������������Կ����еĶ�����̼��ˮ�����Ƚ������ܣ����²������ƫ�ߣ�����װ���еĶ�����̼û����ȫ�������ܣ����в��࣬��˻ᵼ�²������ƫ�͡�
��4������̼�����Ʋ��ȶ��������ֽ�����̼���ơ�ˮ��CO2���ݴ�Ҳ���Բ���̼�����Ƶĺ��������Է����ĵ�ʵ��ԭ����2NaHCO3 Na2CO3��H2O��CO2����
Na2CO3��H2O��CO2����
���㣺�����Ȼ�����Ʒ��̼�����ƺ����ⶨ��ʵ�鷽�����������

���ⶨ�������Ȼ��Ƶ�С�մ��̬��Ʒ��NaHCO3�����������ɲ����������ַ�����
����һ��
�� 
��������
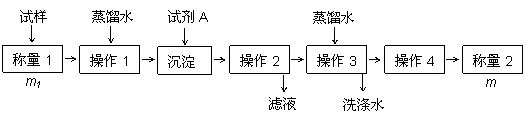 |
�� ��������
 |
��
�����ģ���ʹ�û�ѧ�Լ���ʹ��ʵ���ҳ���������
��Ҫ��ش��������⣺
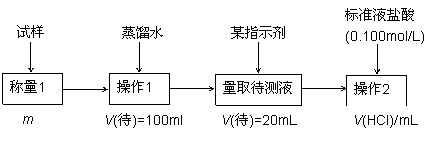
��1������һ�IJ���1��Ҫ�õ��IJ����������ձ���������������Ҫ�������������� ������2���������������������� ���ù���ѡ���ָʾ���������������� ������Ʒ��NaHCO3����������Ϊ������������ [����ͼ������д��m��V(HCl)�ı���ʽ]��
��2��������ѡ��HCO3- ����ת��Ϊ���������أ��Լ�AΪ�������� ���ѧʽ����Һ������3�������������� ��
����ж����Ŀ���Ѿ��ﵽ���������������������������������� ������������
��3�����ݷ����������õ�ʵ��װ�ã����ж���ⶨ��ʵ�������������������� ����ϸ������ʵ��װ�ã��ɴ˲�õ����ݼ������ʵ�����п���ƫ��Ҳ�п���ƫ�͡�
ƫ�ߵ�ԭ������������������������������������������������� ��
ƫ�͵�ԭ������������������������������������������������� ��
��4�������ĵ�ʵ��ԭ�������������������������������������������� ���û�ѧ����ʽ��ʾ����