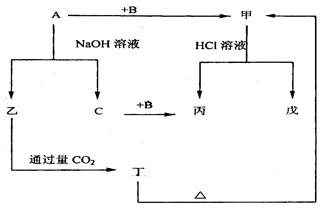
��Ŀ����
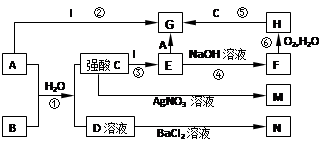
��ͼA��J�������������ˮ��Һ������B��D��G�ǵ��ʣ�B�ǵؿ��к�����ߵĽ���Ԫ�أ�G�����壬J�Ǵ��Բ��ϣ�������HΪ��ɫҺ�塣
����ͼʾ�ش����⣺
(1)д���������ʵĻ�ѧʽ��B________��E________��I________��
(2)д����ӦA��B��E��D��һ����;__________________��
(3)��Ӧ�ٵ����ӷ���ʽ��_________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��__________________________________________��
(4)J�����ᷴӦ�Ļ�ѧ����ʽ��__________________ _________________��
��Ӧ�����Һ��D��Ӧ�����ӷ���ʽ��___________________________________��

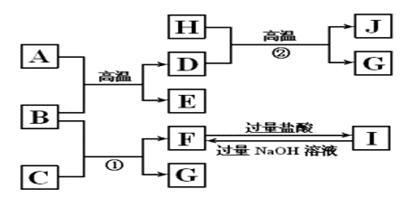
����ͼʾ�ش����⣺
(1)д���������ʵĻ�ѧʽ��B________��E________��I________��
(2)д����ӦA��B��E��D��һ����;__________________��
(3)��Ӧ�ٵ����ӷ���ʽ��_________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��__________________________________________��
(4)J�����ᷴӦ�Ļ�ѧ����ʽ��__________________ _________________��
��Ӧ�����Һ��D��Ӧ�����ӷ���ʽ��___________________________________��
��16��
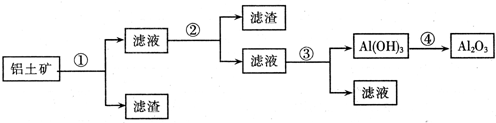
(1)��Al��Al2O3��AlCl3��ÿ��2�ֹ�6�֣�
(2) (1)���Ӹֹ�(������������) ��2�֣�
(3)2Al��2OH����2H2O===2AlO2����3H2�� ��2�֣�
3Fe��4H2O(g) Fe3O4��4H2 ��2�֣�
Fe3O4��4H2 ��2�֣�
(4)Fe3O4��8HCl===FeCl2��2FeCl3��4H2O ��2�֣�
2Fe3����Fe===3Fe2�� ��2�֣�
(1)��Al��Al2O3��AlCl3��ÿ��2�ֹ�6�֣�
(2) (1)���Ӹֹ�(������������) ��2�֣�
(3)2Al��2OH����2H2O===2AlO2����3H2�� ��2�֣�
3Fe��4H2O(g)
 Fe3O4��4H2 ��2�֣�
Fe3O4��4H2 ��2�֣�(4)Fe3O4��8HCl===FeCl2��2FeCl3��4H2O ��2�֣�
2Fe3����Fe===3Fe2�� ��2�֣�
��������� B�ǵؿ��к�����ߵĽ���Ԫ�أ�B��Al��J�Ǵ��Բ���Fe3O4��D�ǵ��ʣ�D��Fe��HΪ��ɫҺ�壬H��H2O, G�ǵ��ʣ�G��H2��A��Fe2O3��E��Al2O3��C��NaOH��F��NaAlO2,I��AlCl3��
(1) B��Al��E��Al2O3��I��AlCl3��
(2)���û���������;�Ǻ��Ӹֹ졣
(3) ��Ӧ����Al��NaOH��Ӧ����H2�����ӷ���ʽ��2Al��2OH����2H2O===2AlO2����3H2������Ӧ��Fe��ˮ�����ķ�Ӧ������ʽ��3Fe��4H2O(g)
 Fe3O4��4H2��
Fe3O4��4H2��(4) J�� Fe3O4������ķ�Ӧ����ʽ��Fe3O4��8HCl===FeCl2��2FeCl3��4H2O��
Fe�ܻ�ԭFe3+�������ӷ���ʽ��2Fe3����Fe===3Fe2����
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ�ֱ�ӵó����ۣ�Ȼ������˳���������������м��ƣ���һ�����������ۡ�

��ϰ��ϵ�д�
�����Ŀ