��Ŀ����
Ϊ�ⶨһ�ָ����������͵Ĵ��Է�ĩ����ɣ���ȡ12.52 g��Ʒ������ȫ�����ڹ���ϡ��������100 mL��Һ��ȡ��һ�룬�������K2SO4��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ���ɵ�4.66 g���塣�����µ�50 mL��Һ��������KSCN��Һ���Ժ�ɫ������������NaOH��Һ�������ɺ��ɫ���������������ˡ�ϴ�ӡ����ȵ�3.2 g���塣��1��������Է�ĩ��������Ԫ�ص�����������
��2��ȷ���ò��ϵĻ�ѧʽ��
��1��20.45% ��2��BaFe2O4
��������ʵ���֪�ø��ϲ����к���Ba��Fe��O����Ԫ�أ�������Ba2+����Ϊx����
Ba2++![]() ====BaSO4��
====BaSO4��
137 233
![]() x 4.66 g
x 4.66 g
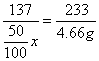 x=5.48 g
x=5.48 g
�躬��Ԫ�ص�����Ϊy����
2Fe �� 2Fe��OH��3 �� Fe2O3
112 160
![]() 3.2 g
3.2 g
 y=4.48 g
y=4.48 g
����Ԫ�ص�����Ϊ��
12.52 g-4.48 g-5.48 g=2.56 g
(1)w(O)=![]() ��100%=20.45%
��100%=20.45%
(2)n(Ba)��n(Fe)��n(O)=![]() =1��2��4
=1��2��4
�ʻ�ѧʽΪ��BaFe2O4��

��ϰ��ϵ�д�
�����Ŀ