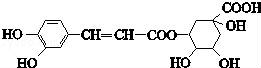��Ŀ����
�ش��������⣺
(1)д����ĭ����������ԭ�������ӷ���ʽ
(2)��֪25�棬101 kPaʱ��
S(s)��O2(g)==SO2(g) ∆H��-296.8 kJ/mol
2Cu(s)��O2(g)==2CuO(s) ∆H��-314.6 kJ/mol
Cu(s)��S(s)==CuS(s) ∆H��-53.1 kJ/mol
д��CuS(s)��O2(g)��Ӧ����CuO(s)��SO2(g)���Ȼ�ѧ����ʽ ��
(3)��֪������AI(OH)3��Ksp=l.0��10-33������Һ��c(Al3+)Ϊ1.0 mol/L�������Al3+��ʼ������pH= �������£�Ũ�Ⱦ�Ϊ0.01mol/L��CH3COOH��CH3COONa�Ļ��Һ�У�PH = a�������ĵ��볣��ԼΪKa = ��
(4)�ڳ����£���V L pH=12��Ba(OH)2��Һ����μ���һ��Ũ�ȵ�NaHSO4ϡ��Һ������Һ�е�Ba2+ ǡ�ó�����ȫʱ����ҺpH=11����Ba(OH)2��Һ��NaHSO4��Һ�������Ϊ ��NaHSO4��Һ�����ʵ���Ũ��Ϊ mol/L��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


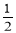 O2(g)=H2O(l) ��H3 = -285.8KJ/mol
O2(g)=H2O(l) ��H3 = -285.8KJ/mol O2(g)=CO2(g)����H1 C(s)��O2(g)=CO(g)����H2
O2(g)=CO2(g)����H1 C(s)��O2(g)=CO(g)����H2 aO(s)��CO2(g)��H7 CaO(s)��H2O(l)=Ca(OH)(s)����H8
aO(s)��CO2(g)��H7 CaO(s)��H2O(l)=Ca(OH)(s)����H8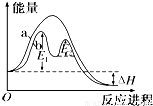
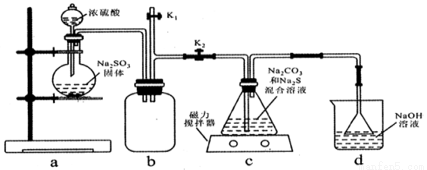
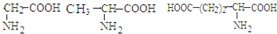 ���ְ�������ˮ���������� 6 �ֶ���
���ְ�������ˮ���������� 6 �ֶ���