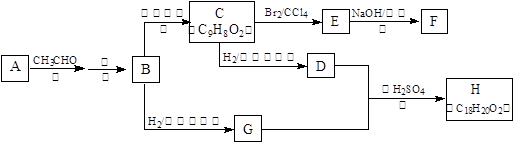��Ŀ����
(12�֣���֪ȩ��һ�������¿��Է�������ת����

����B��һ�ֿ�����Ϊҩ��ķ����廯����������ͼ(���������������ȥ)�и��л����ת���ϵ�ش����⣺

��1��A��B�Ľṹ��ʽΪ��A__________________��B_________________��
��2��G��D��Ӧ����H�Ļ�ѧ����ʽ�ǣ�___________________________��
��3��һ�������£��ܹ���1mol F������Ӧ��H2����������ǣ�_______ mol��
��4��G�ж���ͬ���칹�壬������������Ʒ�Ӧ�ұ�����ֻ��һ��ȡ������ͬ���칹��Ľṹ��ʽΪ������G)��_______________��_________________��__________________��__________________��
���𰸡�
��12�֣���1��A  ��2������ B
��2������ B  ��2����
��2����
��2�� ��
�� 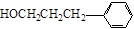

 �� H2O��2����
�� H2O��2����
��3��5 ��2����
��4��
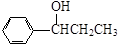

 ��ÿ��1����
��ÿ��1����
��������

��ϰ��ϵ�д�
�����Ŀ



 +NaOH��
+NaOH�� +H2O
+H2O +3Br2��
+3Br2�� ��+3HB
��+3HB