��Ŀ����
���л���ѧ������
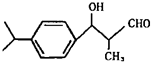
��.���ǻ��������һ��ǿЧ�ĵ�����ϣ���Һ����ʾ����ҵ�н������о��㷺����ṹ��ʽ��ͼ��ʾ��
��1�����л�����еĹ����������� ��д�����е����֣�
��2�����л����ܷ����ķ�Ӧ������(��д����) ��
A��������Ӧ B����ȥ��Ӧ C���ӳɷ�Ӧ D��ˮ�ⷴӦ
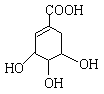
��.�����廯����C10H10O2�����µ�ת����ϵ��
��֪E��ʹBr2/CCl4��Һ��ɫ����ش��������⣺
��3����д��A�Ľṹ��ʽ�� ��
��4�����л���F��C��Ϊͬ���칹�壬�����л���B��Ϊͬϵ���F��ͬ���칹����
�֡�
��5����д��B��C��Ӧ�Ļ�ѧ��Ӧ����ʽ ��
��1�����ǻ������ǻ������Ȼ���̼̼˫�� ��2�֣�
��2��A��C ��2�֣�
��3�� ��2�֣�
��4��3 ��2�֣�
��5��CH2��CHCOOH��CH3OH H2O ��CH2��CHCOOCH3 ��2�֣�
����:��

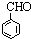 B��
B��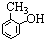 C��
C��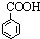 D��
D��
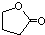 +H2O
+H2O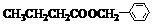

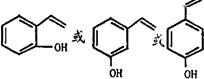
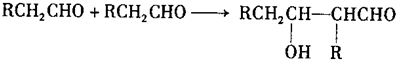
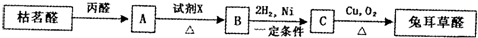

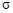 ����
����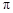 ���ĸ�����Ϊ
���ĸ�����Ϊ 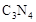 �γɵ�ԭ�Ӿ��壬�ṹ����
�γɵ�ԭ�Ӿ��壬�ṹ����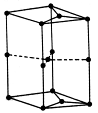
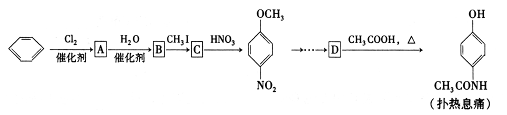
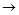 C�ķ�Ӧ����Ϊ ��D�й����ŵ�����Ϊ ��
C�ķ�Ӧ����Ϊ ��D�й����ŵ�����Ϊ �� ��Һ��Ӧ������������
��Һ��Ӧ������������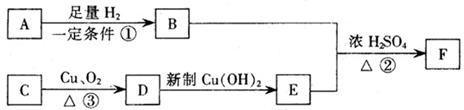
 �����䷴Ӧ������ ��
�����䷴Ӧ������ ��