��Ŀ����
ij��ѧ��ȤС��Ϊ�ⶨFeCl3��Ʒ��������FeCl2���ʣ�����Ԫ�ص�������������ʵ���Ұ����²������ʵ�飺
�ٳ�ȡa g��Ʒ�������ձ��У�
�ڼ������������������ˮʹ��Ʒ�ܽ⣬ȷ���Ƴ�250mL��Һ��
��ȷ��ȡ25.00mL������������Ƶ���Һ�������ձ��У�����������ˮ��ʹ��Ӧ��ȫ��
�ܼ��������ˮ����ֽ��裬ʹ������ȫ��
�ݹ��ˣ�ϴ�ӳ�����
������ת�Ƶ������ڣ����ȡ����裬ֱ�������ɺ��ɫȫ����Ϊ����ɫ���ڸ���������ȴ�����º�����
�ߡ���
����������������ش�
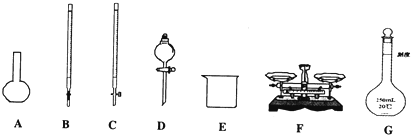
��1������٢ڢ��б����õ�E�� ����������������������ѡ����������ţ���
��2��������з�����Ӧ�����ӷ���ʽΪ ��
��3��������з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4���ڢ��IJ����У�����������ȣ���ȴ�����£�����������Ϊm1g���ٴμ��Ȳ���ȴ�����³���������Ϊm2g����m1��m2��ֵ�ϴ������IJ���Ӧ���� ��
��5��������������W1g�����������������������W2g������Ʒ����Ԫ�ص���������Ϊ ����������ʵ���������Ԫ�ص���ʧ��
�ٳ�ȡa g��Ʒ�������ձ��У�
�ڼ������������������ˮʹ��Ʒ�ܽ⣬ȷ���Ƴ�250mL��Һ��
��ȷ��ȡ25.00mL������������Ƶ���Һ�������ձ��У�����������ˮ��ʹ��Ӧ��ȫ��
�ܼ��������ˮ����ֽ��裬ʹ������ȫ��
�ݹ��ˣ�ϴ�ӳ�����
������ת�Ƶ������ڣ����ȡ����裬ֱ�������ɺ��ɫȫ����Ϊ����ɫ���ڸ���������ȴ�����º�����
�ߡ���
����������������ش�

��1������٢ڢ��б����õ�E��
��2��������з�����Ӧ�����ӷ���ʽΪ
��3��������з�����Ӧ�Ļ�ѧ����ʽΪ
��4���ڢ��IJ����У�����������ȣ���ȴ�����£�����������Ϊm1g���ٴμ��Ȳ���ȴ�����³���������Ϊm2g����m1��m2��ֵ�ϴ������IJ���Ӧ����
��5��������������W1g�����������������������W2g������Ʒ����Ԫ�ص���������Ϊ
��������1�����ݳ������ܽ⡢����һ�����ʵ���Ũ�ȵ���Һ��ȷ��ȡ��Һȷ������������
��2��������ˮ�ܽ�FeCl2��������FeCl3��
��3���Ȼ�����Һ����һˮ�ϰ���Ӧ�������������������Ȼ�泥�
��4�����ݳ��������ʵ������dz��������صķ���������
��5��������Ԫ�������غ㣬������ɫ���壨 Fe2O3���е���������Ʒ�������������������Ĺ�ʽ�����Ԫ�ص�����������
��2��������ˮ�ܽ�FeCl2��������FeCl3��
��3���Ȼ�����Һ����һˮ�ϰ���Ӧ�������������������Ȼ�泥�
��4�����ݳ��������ʵ������dz��������صķ���������
��5��������Ԫ�������غ㣬������ɫ���壨 Fe2O3���е���������Ʒ�������������������Ĺ�ʽ�����Ԫ�ص�����������
����⣺��1�������ʱ��Ҫ��ƽ��Կ�ף��ܽ�ʱ��Ҫ�ձ���������������һ�����ʵ���Ũ�ȵ���Һʱ��Ҫ��Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��ȷ��ȡ��Һȷ��������ʽ�ζ��ܡ�ϴ���ʴ�Ϊ��CFG��
��2������ˮ�ܽ�FeCl2��������FeCl3��2Fe2++Cl2=2Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2=2Fe3++2Cl-��
��3���Ȼ�����Һ����һˮ�ϰ���Ӧ�������������������Ȼ�泥���Ӧ�Ļ�ѧ����ʽΪ��FeCl3+3NH3?H2O=Fe��OH��3��+3NH4Cl��
�ʴ�Ϊ��FeCl3+3NH3?H2O=Fe��OH��3��+3NH4Cl��
��4���ڢ��IJ����У�����������ȣ���ȴ�����£�����������Ϊm1g���ٴμ��Ȳ���ȴ�����³���������Ϊm2g����m1��m2��ֵ�ϴ������IJ���Ӧ���Ǽ������ȣ����ø���������ȴ��������������γ���������������0.1gΪֹ��
�ʴ�Ϊ���������ȣ����ø���������ȴ��������������γ���������������0.1gΪֹ��
��5������Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3����Ԫ�ص�����Ϊ��W2-W1��g��
����Ʒ����Ԫ�ص�����������
=
%��
�ʴ�Ϊ��
%��
��2������ˮ�ܽ�FeCl2��������FeCl3��2Fe2++Cl2=2Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2=2Fe3++2Cl-��
��3���Ȼ�����Һ����һˮ�ϰ���Ӧ�������������������Ȼ�泥���Ӧ�Ļ�ѧ����ʽΪ��FeCl3+3NH3?H2O=Fe��OH��3��+3NH4Cl��
�ʴ�Ϊ��FeCl3+3NH3?H2O=Fe��OH��3��+3NH4Cl��
��4���ڢ��IJ����У�����������ȣ���ȴ�����£�����������Ϊm1g���ٴμ��Ȳ���ȴ�����³���������Ϊm2g����m1��m2��ֵ�ϴ������IJ���Ӧ���Ǽ������ȣ����ø���������ȴ��������������γ���������������0.1gΪֹ��
�ʴ�Ϊ���������ȣ����ø���������ȴ��������������γ���������������0.1gΪֹ��
��5������Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3����Ԫ�ص�����Ϊ��W2-W1��g��
| 112 |
| 160 |
| 112(W2-W1) |
| 160a |
| 700(W2-W1) |
| a |
�ʴ�Ϊ��
| 700(W2-W1) |
| a |
������������Ҫ��������Ԫ�ص����������IJⶨ��ͬʱ������ʵ��֪ʶ���ѶȲ���

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ