��Ŀ����
��16�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺
��1����ͬѧ�����Fe��Cu�ֱ���S��Cl2��Ӧ��ʵ�飬���������в�����Ϊ��ͬѧʵ��õ�����������
A��FeCl3 B��FeCl2 C��CuCl2 D��FeS
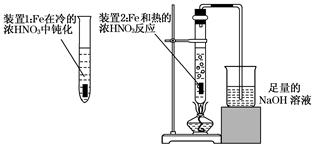
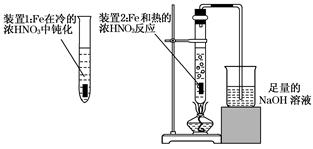
��2����ͬѧΪ��֤Fe�ܺ��ȵ�ŨHNO3��Ӧ�����������ͼ��ʾ��ʵ��װ�ã���˵��װ��B�����ã� ����ʼ����ǰ ����С����ޡ�����������
��3������ͬѧʵ�����ʱ������ȫ�ܽ⣬�Թ�Һ���Ϸ�Ϊ��ɫ���壬�Թ��ϲ�Ϊ����ɫ���壬��ʱ��ͬѧ�����õ���Һ��������ʵ���̽����
�������õ���Һ�м���һС��CuƬ�����CuƬ�����ܽ⣬��������������Ӧһ��ʱ������ܽ⡣��ͬѧ���ݷ�Ӧ����ó�CuƬ�ܽ��ԭ������Ǻ�����HNO3������Ӧ�������ݵķ�Ӧ������ ���÷�Ӧ�����ӷ���ʽΪ ������ΪCuƬ�ܽ��Ƿ�����һԭ���������û�ѧ����ʽ��ʾ�����ɣ� �����˿ղ��
���������ʵ��֤���ڼ���CuƬ����Ӧ��ȫ�����Һ�к���Fe2����������Fe3����˵������IJ�����ʵ������ ��
�۱�ͬѧʵ��������ˮϡ�ͺ�õ���Һ500mL������ʵ��ȫ��������ֻ����ԭ��NO��
NO2��0.02 mol�������Һ��Fe2����Cu2+Ũ�Ⱦ�Ϊ0.02 mol/L����NO3-�����ʵ���Ũ��Ϊ
mol/L������NO��������Ϊ_________________L����״������
��4������̽��ʵ���õ��ܶ�Ϊ1.5g��cm-3��������Ϊ95%��Ũ����3mL������ʵ�ʲμӷ�Ӧ������ԭ���У�д�����㣩���� ��
��1��B��1�֣�
��2���ٻ������âڷ��������ã���1�֣��д������һ���1�֣��ޣ���1�֣�
��3����������ɫ���壻�Թ��ϲ����ֺ���ɫ���壨1�֣���
3Cu+2NO3-+8 H+=3Cu2++2NO��+4 H2O (2��)��2Fe(NO3)3+Cu=Cu(NO3)2+2Fe(NO3)2��2�֣���
��ȡ����Һ�������μ�������KSCN��Һ�������ֺ�ɫ���ٵμ�����������ˮ�����ֺ�ɫ��2�֣�
��0.08����2�֣�0.224����2�֣�
��4����Ũ�����ӷ���������ӷ���1�֣����������ȷֽ⡣��1�֣�
����:��

��16�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺
��1����ͬѧ�����Fe��Cu�ֱ���S��Cl2��Ӧ��ʵ�飬���������в�����Ϊ��ͬѧʵ��õ�����������
| A��FeCl3 | B��FeCl2 | C��CuCl2 | D��FeS |
��3������ͬѧʵ�����ʱ������ȫ�ܽ⣬�Թ�Һ���Ϸ�Ϊ��ɫ���壬�Թ��ϲ�Ϊ����ɫ���壬��ʱ��ͬѧ�����õ���Һ��������ʵ���̽����
�������õ���Һ�м���һС��CuƬ�����CuƬ�����ܽ⣬��������������Ӧһ��ʱ������ܽ⡣��ͬѧ���ݷ�Ӧ����ó�CuƬ�ܽ��ԭ������Ǻ�����HNO3������Ӧ�������ݵķ�Ӧ������ ���÷�Ӧ�����ӷ���ʽΪ ������ΪCuƬ�ܽ��Ƿ�����һԭ���������û�ѧ����ʽ��ʾ�����ɣ� �����˿ղ��
���������ʵ��֤���ڼ���CuƬ����Ӧ��ȫ�����Һ�к���Fe2����������Fe3����˵������IJ�����ʵ������ ��
�۱�ͬѧʵ��������ˮϡ�ͺ�õ���Һ500mL������ʵ��ȫ��������ֻ����ԭ��NO��NO2��0.02 mol�������Һ��Fe2����Cu2+Ũ�Ⱦ�Ϊ0.02 mol/L����NO3-�����ʵ���Ũ��Ϊ
mol/L������NO��������Ϊ _________________L����״������
��4������̽��ʵ���õ��ܶ�Ϊ1.5g��cm-3��������Ϊ95%��Ũ����3mL������ʵ�ʲμӷ�Ӧ������ԭ���У�д�����㣩���� ��
��16�֣�Fe��Cu�����������ʹ�õĽ�����ijУ��ѧ�о���ѧϰС���ͬѧ����ʵ����ֶ��о�Fe��Cu�Լ��������������ʡ���������о����ش��������⣺
��1����ͬѧ�����Fe��Cu�ֱ���S��Cl2��Ӧ��ʵ�飬���������в�����Ϊ��ͬѧʵ��õ�����������
| A��FeCl3 | B��FeCl2 | C��CuCl2 | D��FeS |
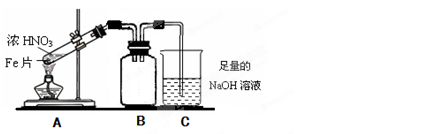
��2����ͬѧΪ��֤Fe�ܺ��ȵ�ŨHNO3��Ӧ�����������ͼ��ʾ��ʵ��װ�ã���˵��װ��B�����ã� ����ʼ����ǰ ����С����ޡ�����������
��3������ͬѧʵ�����ʱ������ȫ�ܽ⣬�Թ�Һ���Ϸ�Ϊ��ɫ���壬�Թ��ϲ�Ϊ����ɫ���壬��ʱ��ͬѧ�����õ���Һ��������ʵ���̽����
�������õ���Һ�м���һС��CuƬ�����CuƬ�����ܽ⣬��������������Ӧһ��ʱ������ܽ⡣��ͬѧ���ݷ�Ӧ����ó�CuƬ�ܽ��ԭ������Ǻ�����HNO3������Ӧ�������ݵķ�Ӧ������ ���÷�Ӧ�����ӷ���ʽΪ ������ΪCuƬ�ܽ��Ƿ�����һԭ���������û�ѧ����ʽ��ʾ�����ɣ� �����˿ղ��
���������ʵ��֤���ڼ���CuƬ����Ӧ��ȫ�����Һ�к���Fe2����������Fe3����˵������IJ�����ʵ������ ��
�۱�ͬѧʵ��������ˮϡ�ͺ�õ���Һ500mL������ʵ��ȫ��������ֻ����ԭ��NO��NO2��0.02 mol�������Һ��Fe2����Cu2+Ũ�Ⱦ�Ϊ0.02 mol/L����NO3-�����ʵ���Ũ��Ϊ
mol/L������NO��������Ϊ _________________L����״������
��4������̽��ʵ���õ��ܶ�Ϊ1.5g��cm-3��������Ϊ95%��Ũ����3mL������ʵ�ʲμӷ�Ӧ������ԭ���У�д�����㣩���� ��