��Ŀ����
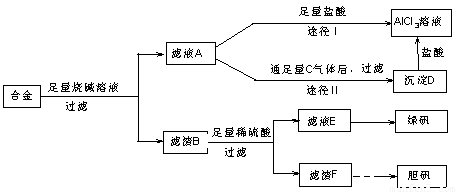
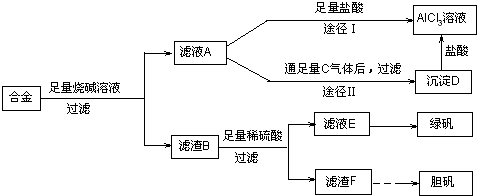
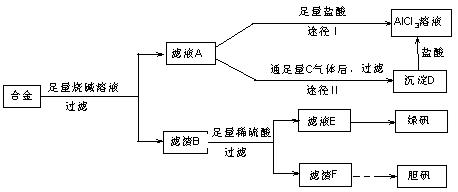
��18�֣�ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����[FeSO4��7H2O]�͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�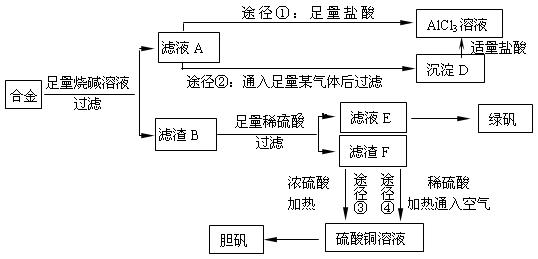
�ش��������⣺
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ ��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�����Ϊ������ �� ������ʵ�鷽���ദ�����˹��˲������������õ��IJ��������� �Ͳ����������в������������� ��
�� ������ʵ�鷽���ദ�����˹��˲������������õ��IJ��������� �Ͳ����������в������������� ��
��3��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��� �� ��
��4��ͨ��;�� ����ȡ������������е�ʵ��������裺�����ᡢ����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ������С�����ͨ���������������Ϊ �������ӷ���ʽ��ʾ����
����ȡ������������е�ʵ��������裺�����ᡢ����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ������С�����ͨ���������������Ϊ �������ӷ���ʽ��ʾ���� ��5���ⶨ���õ�����CuSO4��xH2O����xֵ��ʵ�鷽������������ͭ�����е�ˮ�õ���ɫ����ˮ����ͭ����ȴ��������˱仯�Ļ�ѧ����ʽΪ��CuSO4��xH2O===C
��5���ⶨ���õ�����CuSO4��xH2O����xֵ��ʵ�鷽������������ͭ�����е�ˮ�õ���ɫ����ˮ����ͭ����ȴ��������˱仯�Ļ�ѧ����ʽΪ��CuSO4��xH2O===C uSO4+xH2O �����¶ȹ��ߣ�CuSO4������ֽ�ΪCuO��SO3���ڴ�ʵ������У������������ٽ��� �Ρ����ⶨ���xֵƫ�ߣ����ܵ�ԭ���� ��
uSO4+xH2O �����¶ȹ��ߣ�CuSO4������ֽ�ΪCuO��SO3���ڴ�ʵ������У������������ٽ��� �Ρ����ⶨ���xֵƫ�ߣ����ܵ�ԭ���� ��
a�������¶ȹ��� b������ʱ��������ɽ�����
c�����Ⱥ���ڿ�������ȴ d��������������δ�����ʪ��
��1��2Al+2OH��+2H2O��2AlO2�� +3H2�� ��2�֣���
��2��;���ڣ�2�֣���  �ձ���©����2�֣��� ������1�֣�
�ձ���©����2�֣��� ������1�֣�
��3��������������;�������������٣�1�֣� ;���ܲ��������Ⱦ���������壨1�֣�
��4������Ũ����1�֣� ���ˣ�1�֣� 2Cu+O2+4H+��2Cu2++2H2O��2�֣�
��5��4��2�֣� abd��3�֣�
����

 ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
 ��
��