��Ŀ����
�������ӷ���ʽ��������ʵ�������ȷ����( )
| A��Ca(HCO3)2��Һ�м�������NaOH��Һ��Ca2++2HCO3-+2OH-=CaCO3��+CO32-+H2O |
| B��������Һ�м��������Ba(OH)2��Һ��Al3++2SO42-+2Ba2++4OH-=A1O2-+2BaSO4+2H2O |
| C������������Һ�м��������ˮ��Al3++4NH3��H2O=A1O2-+4NH4++2H2O |
| D����������������ϡ���Fe3O4+8H++NO3-=3Fe3++NO��+4H2O |
B
���������A���������������Ƶ���̼�������Һ�У�̼����Ʋ���Ҫ���㶨��ɶ��ɣ�����B���������������� ��������Al3+��SO42-���㶨��ɶ��ɣ��ԣ�C����ˮ�����ܽ���������������D�������������غ㡢����غ�͵�ʧ�����غ㣬����

��ϰ��ϵ�д�
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
 Cl2��+H2��+ 2OH-
Cl2��+H2��+ 2OH- H3O++S2-
H3O++S2- + CO2 +H2O��
+ CO2 +H2O��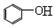 + HCO3-
+ HCO3-

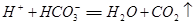
 ��ϡ
��ϡ ��Ӧ��
��Ӧ��