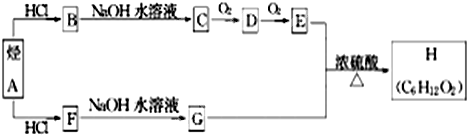
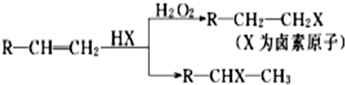
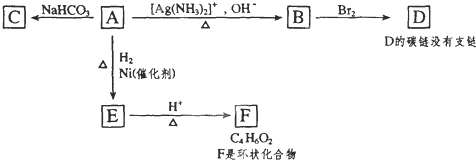
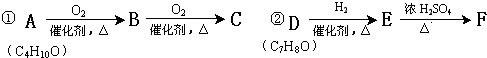��Ŀ����
��A��E��Ϊ���������л������֮��������ת����ϵ��
ϩ��A���ܶ�����ͬ�����������ܶȵ�1.75�����ṹ��������Aû��˳���칹�壻A�ĺ˴Ź�������������壬�����֮��Ϊ1��3��B��һ������������NaOH��Ӧ��D��NaHCO3��Ӧ�ų���ɫ��ζ�����壻E��һ����ˮ����������ɫҺ�壬�㷺�������㽶�����ѡ����ܵ�ˮ���Լ�ơ�ƻ��У���һ����Ҫ�����ϣ�
��1��D�Ľṹ��ʽΪ______
��2��B��һ�������±�ΪA�Ļ�ѧ����ʽΪ______��
��3��A��C�ķ�Ӧ����______
��4��A��X��Ϊͬ���칹�壬���Ǿ�����ͬ�����ţ�X���������仯��������J��J����ˮ���۳ɸ߷��ӻ�����K��
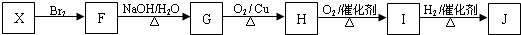
�ٻ�����X��������______
��д���ṹ��ʽ��I______��
����J����K�Ļ�ѧ����ʽΪ______
��F�ж���ͬ���칹�壬���н�����һ������̼�Ľṹ��______��
ϩ��A���ܶ�����ͬ�����������ܶȵ�1.75�����ṹ��������Aû��˳���칹�壻A�ĺ˴Ź�������������壬�����֮��Ϊ1��3��B��һ������������NaOH��Ӧ��D��NaHCO3��Ӧ�ų���ɫ��ζ�����壻E��һ����ˮ����������ɫҺ�壬�㷺�������㽶�����ѡ����ܵ�ˮ���Լ�ơ�ƻ��У���һ����Ҫ�����ϣ�
��1��D�Ľṹ��ʽΪ______
��2��B��һ�������±�ΪA�Ļ�ѧ����ʽΪ______��
��3��A��C�ķ�Ӧ����______
��4��A��X��Ϊͬ���칹�壬���Ǿ�����ͬ�����ţ�X���������仯��������J��J����ˮ���۳ɸ߷��ӻ�����K��
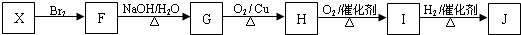
�ٻ�����X��������______
��д���ṹ��ʽ��I______��
����J����K�Ļ�ѧ����ʽΪ______
��F�ж���ͬ���칹�壬���н�����һ������̼�Ľṹ��______��

ϩ��A���ܶ�����ͬ�����������ܶȵ�1.75����A����Է�������Ϊ32��1.75=56����������ΪCxHy����
=4��8������A�ķ���ʽΪC4H8��Aû��˳���칹�壬A�ĺ˴Ź�������������壬�����֮��Ϊ1��3����AΪ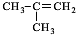 ��D��NaHCO3��Ӧ�ų���ɫ��ζ�����壬D�����Ȼ�-COOH��E��һ����ˮ����������ɫҺ�壬EΪ������ת����ϵ��֪CΪ����B��һ������������NaOH��Ӧ����BΪ±��������BΪ
��D��NaHCO3��Ӧ�ų���ɫ��ζ�����壬D�����Ȼ�-COOH��E��һ����ˮ����������ɫҺ�壬EΪ������ת����ϵ��֪CΪ����B��һ������������NaOH��Ӧ����BΪ±��������BΪ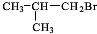 �ȣ�CΪ
�ȣ�CΪ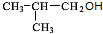 ��DΪ
��DΪ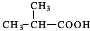 ��EΪ
��EΪ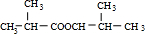 ��
��
��1��������������֪��DΪ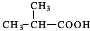 ���ʴ�Ϊ��
���ʴ�Ϊ��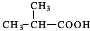 ��
��
��2��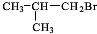 ���������ƴ���Һ�����������·�����ȥ��Ӧ����
���������ƴ���Һ�����������·�����ȥ��Ӧ����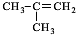 ����Ӧ����ʽΪ��
����Ӧ����ʽΪ��
 ��
��
�ʴ�Ϊ�� ��
��
��3��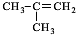 ��ˮ�ų��ӳɷ�Ӧ����
��ˮ�ų��ӳɷ�Ӧ����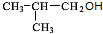 ��
��
�ʴ�Ϊ���ӳɷ�Ӧ��
��4��A��X��Ϊͬ���칹�壬���Ǿ�����ͬ�����ţ���ת����ϵ��֪��FΪ������飨��ԭ�Ӵ�������̼ԭ���ϣ���GΪ��Ԫ�����ǻ���������̼ԭ���ϣ�����G��������H��H��������I��H����-CHO��I����-COOH����X��˫�����ڶ�λ�ã���XΪCH3CH2CH=CH3��FΪCH3CH2CHBrCH2Br��GΪCH3CH2CH��OH��CH2OH��HΪ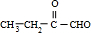 ��IΪ
��IΪ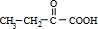 ��JΪ
��JΪ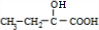 ��J����ˮ���۳ɸ߷��ӻ�����K��KΪ
��J����ˮ���۳ɸ߷��ӻ�����K��KΪ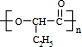 ��
��
��������������֪��������XΪΪCH3CH2CH=CH3�������� 1-��ϩ���ʴ�Ϊ��1-��ϩ��
��������������֪��IΪ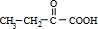 ���ʴ�Ϊ��
���ʴ�Ϊ��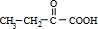 ��
��
��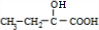 �������۷�Ӧ����
�������۷�Ӧ����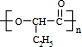 ����Ӧ����ʽΪ��n
����Ӧ����ʽΪ��n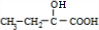
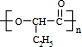 +nH2O��
+nH2O��
�ʴ�Ϊ��n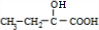
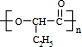 +nH2O��
+nH2O��
��CH3CH2CHBrCH2Br�ж���ͬ���칹�壬���н�����һ������̼�Ľṹ��CH2BrCH2CHBrCH3���ʴ�Ϊ��CH2BrCH2CHBrCH3��
| 56 |
| 12 |
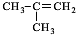 ��D��NaHCO3��Ӧ�ų���ɫ��ζ�����壬D�����Ȼ�-COOH��E��һ����ˮ����������ɫҺ�壬EΪ������ת����ϵ��֪CΪ����B��һ������������NaOH��Ӧ����BΪ±��������BΪ
��D��NaHCO3��Ӧ�ų���ɫ��ζ�����壬D�����Ȼ�-COOH��E��һ����ˮ����������ɫҺ�壬EΪ������ת����ϵ��֪CΪ����B��һ������������NaOH��Ӧ����BΪ±��������BΪ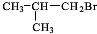 �ȣ�CΪ
�ȣ�CΪ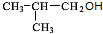 ��DΪ
��DΪ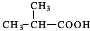 ��EΪ
��EΪ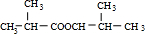 ��
����1��������������֪��DΪ
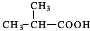 ���ʴ�Ϊ��
���ʴ�Ϊ��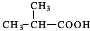 ��
����2��
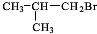 ���������ƴ���Һ�����������·�����ȥ��Ӧ����
���������ƴ���Һ�����������·�����ȥ��Ӧ����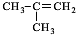 ����Ӧ����ʽΪ��
����Ӧ����ʽΪ�� ��
���ʴ�Ϊ��
 ��
����3��
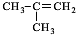 ��ˮ�ų��ӳɷ�Ӧ����
��ˮ�ų��ӳɷ�Ӧ����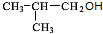 ��
���ʴ�Ϊ���ӳɷ�Ӧ��
��4��A��X��Ϊͬ���칹�壬���Ǿ�����ͬ�����ţ���ת����ϵ��֪��FΪ������飨��ԭ�Ӵ�������̼ԭ���ϣ���GΪ��Ԫ�����ǻ���������̼ԭ���ϣ�����G��������H��H��������I��H����-CHO��I����-COOH����X��˫�����ڶ�λ�ã���XΪCH3CH2CH=CH3��FΪCH3CH2CHBrCH2Br��GΪCH3CH2CH��OH��CH2OH��HΪ
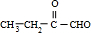 ��IΪ
��IΪ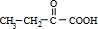 ��JΪ
��JΪ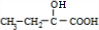 ��J����ˮ���۳ɸ߷��ӻ�����K��KΪ
��J����ˮ���۳ɸ߷��ӻ�����K��KΪ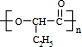 ��
����������������֪��������XΪΪCH3CH2CH=CH3�������� 1-��ϩ���ʴ�Ϊ��1-��ϩ��
��������������֪��IΪ
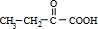 ���ʴ�Ϊ��
���ʴ�Ϊ��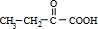 ��
����
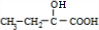 �������۷�Ӧ����
�������۷�Ӧ����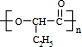 ����Ӧ����ʽΪ��n
����Ӧ����ʽΪ��n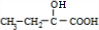
| һ������ |
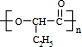 +nH2O��
+nH2O���ʴ�Ϊ��n
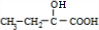
| һ������ |
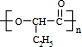 +nH2O��
+nH2O����CH3CH2CHBrCH2Br�ж���ͬ���칹�壬���н�����һ������̼�Ľṹ��CH2BrCH2CHBrCH3���ʴ�Ϊ��CH2BrCH2CHBrCH3��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ��
��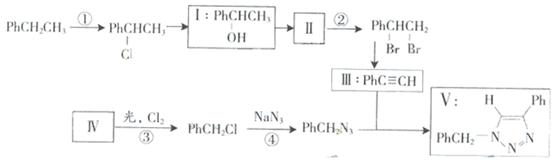
 C��6N2
C��6N2

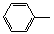 -CH=CH2����D��HO-
-CH=CH2����D��HO-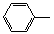 -CHO�������·����ϳɣ�
-CHO�������·����ϳɣ�
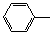 -ONa+RCH2I��
-ONa+RCH2I�� -OCH2R
-OCH2R