��Ŀ����
��6�֣�������������Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⡣���ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��
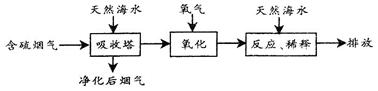
��1����Ȼ��ˮ�ʼ��ԣ�д��SO2��OH-��Ӧ�����ӷ���ʽ�� ��
��2����Ȼ��ˮ�����˺��������������H2SO3���ӣ�ʹ���������������Ļ�ѧԭ����
���û�ѧ����ʽ��ʾ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��

��1����Ȼ��ˮ�ʼ��ԣ�д��SO2��OH-��Ӧ�����ӷ���ʽ�� ��
��2����Ȼ��ˮ�����˺��������������H2SO3���ӣ�ʹ���������������Ļ�ѧԭ����
���û�ѧ����ʽ��ʾ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
��1��SO2+2OH-==SO32-+H2O ��2��2H2SO3+O2==2H2SO4�к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����
���⿼���˹�ҵ�뻯ѧ�����й�ϵ����1��SO2����Ӧ�����κ�ˮ����Ӧ�����ӷ���ʽΪ��SO2+2OH-==SO32-+H2O����2�������������Ҫ�ɷ�ΪSO2,SO2������ˮ������H2SO3��������H2SO3����Ļ��ϼ�Ϊ+4�ۣ���H2SO3���л�ԭ�ԣ��ɱ�������������Ӧ�Ļ�ѧ����ʽΪ2H2SO3+O2==2H2SO4��������Ȼ��ˮ�ʼ��ԣ�������ġ���ˮ����֮��Ͽɷ����кͷ�Ӧ��ϡ�;�������ˮ�����ɵ��ᡣ

��ϰ��ϵ�д�
 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�
�����Ŀ
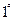 ��ˮ��ָÿ��ˮ��10
��ˮ��ָÿ��ˮ��10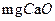 ����֮�൱�����ʣ���7.1
����֮�൱�����ʣ���7.1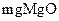 ������ij��Ȼˮ��
������ij��Ȼˮ��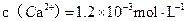 ��
��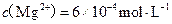 �����ˮ��Ӳ��Ϊ ��
�����ˮ��Ӳ��Ϊ ��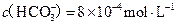 ����Ҫ����10
����Ҫ����10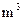 ������Ȼˮ�������ȼ���
������Ȼˮ�������ȼ���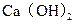
 �������
�������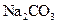
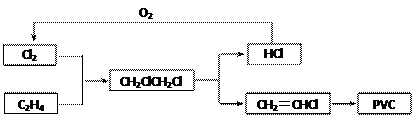
 2Cl2(g)��2H2O(g) ��H����114.4kJ��mol��1��
2Cl2(g)��2H2O(g) ��H����114.4kJ��mol��1��