��Ŀ����
һ�������½�2.3 mol SO2�����1.2 mol O2�������һ�ݻ��ɱ���ܱ������У��ɻ���������λ����ͼ1��ʾ���ں��º�ѹ�·������·�Ӧ�� 2SO2��g��+ O2��g�� 2SO3��g�������С�H < 0 ������Ӧ�ﵽƽ��ʱ������λ����ͼ2��ʾ��
2SO2��g��+ O2��g�� 2SO3��g�������С�H < 0 ������Ӧ�ﵽƽ��ʱ������λ����ͼ2��ʾ��
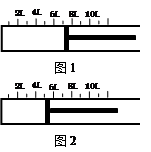
��1��ƽ��ʱSO2ת����Ϊ ����������ȷ��1%����
��2���������·�Ӧ��ƽ�ⳣ��Ϊ ��������λ��Ч���֣���
��3�������¶ȶԸ÷�Ӧ��SO2ת���ʵ�Ӱ�� ��
 2SO2��g��+ O2��g�� 2SO3��g�������С�H < 0 ������Ӧ�ﵽƽ��ʱ������λ����ͼ2��ʾ��
2SO2��g��+ O2��g�� 2SO3��g�������С�H < 0 ������Ӧ�ﵽƽ��ʱ������λ����ͼ2��ʾ��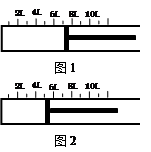
��1��ƽ��ʱSO2ת����Ϊ ����������ȷ��1%����
��2���������·�Ӧ��ƽ�ⳣ��Ϊ ��������λ��Ч���֣���
��3�������¶ȶԸ÷�Ӧ��SO2ת���ʵ�Ӱ�� ��
��1��87% ����2�� 1.11��103����3�������¶ȣ�SO2ת���ʽ���(����������Ҳ��)
������������º�ѹʱ����������֮�ȵ������ʵ���֮�ȣ�ƽ��ʱ�������Ϊ5L�����ʵ���Ϊ2.5 mol,����ƽ������ʽ��2SO2��g��+ O2��g��
 2SO3��g��
2SO3��g����ʼ 2.3 1.2 0
�仯 2X X 2X
ƽ�� 2.3-2X 1.2- X 2X
2.3-2X + 1.2- X + 2X=2.5 X=1
����ƽ��ʱSO2ת����=2/2.3=0.87��ƽ�ⳣ����1.11��103�������¶�,ƽ�������ƶ�,���������ת���ʼ�С.

��ϰ��ϵ�д�
 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
�����Ŀ
 2CO(g)��2H2(g)�����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ����ͼ�������й�˵��һ����ȷ���ǣ� ��
2CO(g)��2H2(g)�����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ����ͼ�������й�˵��һ����ȷ���ǣ� ��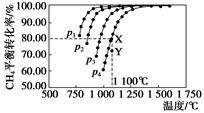
 2C(g)����H<0���ﵽƽ���ֻ�ı�һ������(X)��������(Y)�ı仯һ������ͼ�����ߵ��� (����)
2C(g)����H<0���ﵽƽ���ֻ�ı�һ������(X)��������(Y)�ı仯һ������ͼ�����ߵ��� (����)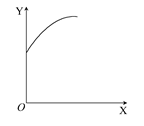
 2SO3������Ӧ���ʷֱ���v��SO2����v��O2����v��SO3����mol��L��1��min��1����ʾ���淴Ӧ���ʷֱ���v�䣨SO2����v�䣨O2����v�䣨SO3����mol��L��1��min��1����ʾ������Ӧ�ﵽ��ѧƽ��ʱ����ȷ�Ĺ�ϵ��
2SO3������Ӧ���ʷֱ���v��SO2����v��O2����v��SO3����mol��L��1��min��1����ʾ���淴Ӧ���ʷֱ���v�䣨SO2����v�䣨O2����v�䣨SO3����mol��L��1��min��1����ʾ������Ӧ�ﵽ��ѧƽ��ʱ����ȷ�Ĺ�ϵ��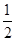 v�䣨SO2��
v�䣨SO2�� 2Z(g)������X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1 mol��L��1��0.3 mol��L��1��0.2 mol��L��1����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п�����(����)��
2Z(g)������X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1 mol��L��1��0.3 mol��L��1��0.2 mol��L��1����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п�����(����)�� TaI4��g����S2��g������H>0��I��
TaI4��g����S2��g������H>0��I��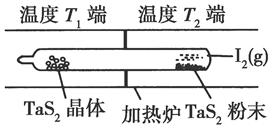
 ��________���������С�����䡱����
��________���������С�����䡱���� p C(g)+q D(g)�ķ�Ӧ�У���5 min�ﵽƽ�⣬���A����3 mol��L-1��B����1 mol��L-1��C����2 mol��L-1����ʱ������ϵ��ѹ��ƽ�ⲻ�ƶ�����m��n��p��qΪ( )
p C(g)+q D(g)�ķ�Ӧ�У���5 min�ﵽƽ�⣬���A����3 mol��L-1��B����1 mol��L-1��C����2 mol��L-1����ʱ������ϵ��ѹ��ƽ�ⲻ�ƶ�����m��n��p��qΪ( )