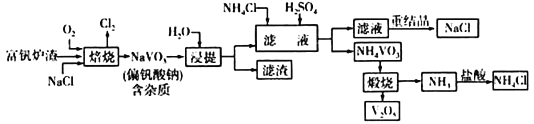
��Ŀ����
����Ŀ����ҵ���Ʊ���ϩ�棨CH2=CHC��N������ɫ��������Ȳ����
��Ȳ����![]()
��1������˵����ȷ����____________��������ѡ��
a. NH4+�ռ乹�ͳ���������
b. CH2=CHCN������ֻ��̼����ԭ��λ��ͬһƽ��
c. C2H2��HCN����������ԭ�Ӿ�λ��ͬһֱ��
d. NH3��������H2O����Ҫ����Ϊ���Ƕ��Ǽ��Է���
��2����NH3��Ϊ�ȵ������������Ϊ______________��д��ѧʽ����
��3��1mol��ϩ������к���![]() ������ĿΪ__________________��
������ĿΪ__________________��
��4��ͭ����Ũ���Ỻ�������û���Ӧ�����������������H[CuCl2]���ɡ��÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
���𰸡� ac H3O+ 6NA 2Cu + 4HCl === 2HCuCl2 + H2 ��
����������1��a��NH4+�е�ԭ���ӻ������=������+�¶Ե��Ӷ���=4+0=4�����Բ�ȡsp3�ӻ������Կռ乹�ͳ��������壬a��ȷ��b��CH2=CH-C��N�����൱��һ��̼̼˫����һ��̼���μ�ͨ��һ��̼̼������������������ԭ�Ӷ�����ͬһƽ���ϣ�b����c��C2H2��HCN���Ӷ�����һ�����������Զ�Ϊsp�ӻ���������ԭ�Ӿ�λ��ͬһֱ�ߣ�c��ȷ��d��NH3�Ǽ��Է��ӣ�ˮ�Ǽ����ܼ������Է��������ڼ����ܼ���ͬʱNH3����H2O���Ӽ��γ���������Ը�����������ԭ������NH3��������H2O��d����ѡac����2��NH3����4��ԭ�ӣ��۵�������Ϊ8�����ӣ����Ӧ�ĵȵ�������H3O+����3��ÿ��������˫��������������1��������ͨ����ϩ��ĽṹCH2��CHC��N������֪����1mol��ϩ������к�����������ĿΪ6NA����4��ͭ����Ũ���Ỻ�������û���Ӧ���������H[CuCl2]���ɣ����Է�Ӧ����ʽΪ��2Cu+4HCl��Ũ��=2H[CuCl2]+H2����

����Ŀ��X��Y��Z��W��Q��RΪԭ���������������ǰ������Ԫ�ء��������Ϣ���±���
Ԫ�ش��� | �����Ϣ |
X | ԭ�Ӻ����������������������� |
Y | ԭ�Ӻ������ռ3����ͬ�ܼ�����ÿ���ܼ����Ų��ĵ�������ͬ |
Z | �����̬�⻯����ʹʪ��ĺ�ɫʯ����ֽ���� |
W | ԭ�ӵ�L���Ӳ�����2�ԳɶԵ��� |
Q | Ԫ�ص��������������۵Ĵ�����Ϊ6 |
R | �������ճ���������;��㡢�������Ľ������� |
��ش��������⣺
��1��1����̬Rԭ�ӵĺ�������Ų�ʽΪ________��R��YW���γ������R(YW)5,��R(YW)5��R �Ļ��ϼ�Ϊ_________��
��2��Y��Z��W����Ԫ�صĵ縺����С�����˳��Ϊ_________����Ԫ�ط��ű�ʾ����
��3����̬Qԭ�ӵ�����ܲ���е�ԭ�ӹ����Ϊ________��
��4�����й���Y2X2��˵������ȷ����______������ĸ����
A. Y2X2�е�����ԭ�Ӷ�����8�����ȶ��ṹ
B��ÿ��Y2X2������������������Ŀ֮��Ϊ1:1
C. Y2X2���ɼ��Լ��ͷǼ��Լ����ɵķǼ��Է���
D. Y2X2�ķ��ӹ���Ϊֱ����
��5��ZQ3����ԭ�ӵ��ӻ��������Ϊ______������ӿռ乹��Ϊ_______��