��Ŀ����
 Fe��OH��2��ˮ���ǰ�ɫ�������ڿ������ױ��������йص�ʵ��������
Fe��OH��2��ˮ���ǰ�ɫ�������ڿ������ױ��������йص�ʵ����������ɫ�����ȱ�ɻ���ɫ������ɺ��ɫ
��ɫ�����ȱ�ɻ���ɫ������ɺ��ɫ
����Ӧ�Ļ�ѧ����ʽ��4Fe��OH��2+O2+2H2O=4Fe��OH��3
4Fe��OH��2+O2+2H2O=4Fe��OH��3
��ʵ���ҿ����������ַ����Ƶð�ɫ��Fe��OH��2����������һ���ò���Fe3+��FeSO4��Һ�벻��O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1����FeSO4��Һ�к���Fe3+���ɼ���Fe��ʹ�仹ԭ����Ӧ�����ӷ���ʽ��
2Fe3++Fe=3Fe 2+
2Fe3++Fe=3Fe 2+
����2����ȥ����ˮ���ܽ��O2������
���
���
�ķ�������3�����ɰ�ɫFe��OH��2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������
�������ɵ�Fe��OH��2�����Ӵ�O2
�������ɵ�Fe��OH��2�����Ӵ�O2
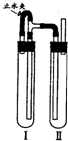
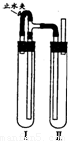
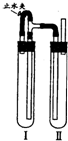
��������������ͼװ���У��Թܢ��������м��ϡH2SO4���Թܢ������NaOH��Һ��Ϊ���Ƶð�ɫFe��OH��2������ʵ��ʱ��ֹˮ�У��������Ӻ��ʵ�鲽����
�����Թܢ���ڴ��ų��������Ĵ��ȣ����ų���H2����ʱ���ټн�ֹˮ��
�����Թܢ���ڴ��ų��������Ĵ��ȣ����ų���H2����ʱ���ټн�ֹˮ��
���������ɵ�Fe��OH��2�����ܽϳ�ʱ�䱣�ְ�ɫ���������������������ȶ����ױ��������������������������������������Ǻ��ɫ������
��ʵ���������ַ������Ʊ�������������
����һ ��ȫ�Dz��ÿα��е�ʵ�飬��������������Һ�������е�Ҫ����Ҫע���ֹˮ������������Ʊ���������������Ҫ��ȥ�ܽ�����Һ�е��������Ʊ������������IJ���Ҫ��
������ �ǶԿα�ʵ������죬��һ�ָĽ����Ʊ����������������������ķ�������֤���Ƶ�������������������������
��ʵ���������ַ������Ʊ�������������
����һ ��ȫ�Dz��ÿα��е�ʵ�飬��������������Һ�������е�Ҫ����Ҫע���ֹˮ������������Ʊ���������������Ҫ��ȥ�ܽ�����Һ�е��������Ʊ������������IJ���Ҫ��
������ �ǶԿα�ʵ������죬��һ�ָĽ����Ʊ����������������������ķ�������֤���Ƶ�������������������������
����⣺�������������ȶ����ױ��������������������������������������Ǻ��ɫ���������Կ����������ǣ���ɫ�����ȱ�ɻ���ɫ������ɺ��ɫ����Ӧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ����ɫ�����ȱ�ɻ���ɫ������ɺ��ɫ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��1�������Ӿ��������ԣ��������������������ӣ����ӷ�Ӧ����ʽΪ��2Fe3++Fe=3Fe 2+��
�ʴ�Ϊ��2Fe3++Fe=3Fe 2+��
��2���������ˮ�ɳ�ȥ�����ܽ��O2���ʴ�Ϊ����У�
��3��Fe��OH��2�����ױ������е�����������ʵ��ʱ���ɰ�ɫFe��OH��2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ���������ɵ� Fe��OH��2�����Ӵ�O2����������������
�ʴ�Ϊ���������ɵ� Fe��OH��2�����Ӵ�O2��
��4����ֹˮ�У�Fe��H2SO4��Ӧ����H2��������װ�ã���Ӧһ��ʱ���ر�ֹˮ�У�����Թ�����ѹ���ߣ���Ӧ���ɵ�Fe2+�ص��ܽ����Ҳ��Թ���NaOH��Ӧ���ɰ�ɫ����Fe��OH��2��������ر�ֹˮ�У�ʹ����Թ��е�����ѹ���Ҳ��Թ��У���NaOH�кͣ���ò���Fe��OH��2��Һ��
�ʴ�Ϊ�������Թܢ���ڴ��ų��������Ĵ��ȣ����ų���H2����ʱ���ټн�ֹˮ�У�
�ʴ�Ϊ����ɫ�����ȱ�ɻ���ɫ������ɺ��ɫ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��1�������Ӿ��������ԣ��������������������ӣ����ӷ�Ӧ����ʽΪ��2Fe3++Fe=3Fe 2+��
�ʴ�Ϊ��2Fe3++Fe=3Fe 2+��
��2���������ˮ�ɳ�ȥ�����ܽ��O2���ʴ�Ϊ����У�
��3��Fe��OH��2�����ױ������е�����������ʵ��ʱ���ɰ�ɫFe��OH��2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ���������ɵ� Fe��OH��2�����Ӵ�O2����������������
�ʴ�Ϊ���������ɵ� Fe��OH��2�����Ӵ�O2��
��4����ֹˮ�У�Fe��H2SO4��Ӧ����H2��������װ�ã���Ӧһ��ʱ���ر�ֹˮ�У�����Թ�����ѹ���ߣ���Ӧ���ɵ�Fe2+�ص��ܽ����Ҳ��Թ���NaOH��Ӧ���ɰ�ɫ����Fe��OH��2��������ر�ֹˮ�У�ʹ����Թ��е�����ѹ���Ҳ��Թ��У���NaOH�кͣ���ò���Fe��OH��2��Һ��
�ʴ�Ϊ�������Թܢ���ڴ��ų��������Ĵ��ȣ����ų���H2����ʱ���ټн�ֹˮ�У�
���������⿼�����ʵ��Ʊ���ע��Fe��OH��2�����ױ������е���������������Fe��OH��2����Ҫ���ʣ���������ԭ�����ʻ����Ͻ����˸ı࣬��Ƴ���̽����ʵ���⣬���⿼������ݱȽ϶࣬����ˮ�ⷽ������⣬����������ԭ��������⣬����ʵ���е�ʵ�����⣬ͬʱ��������ʵ����ƣ��ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ


 Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O
Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O Mn3O4+SO2��+2SO3��+3H2O
Mn3O4+SO2��+2SO3��+3H2O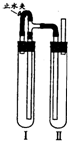 Fe��OH��2��ˮ���ǰ�ɫ�������ڿ������ױ��������йص�ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______��ʵ���ҿ����������ַ����Ƶð�ɫ��Fe��OH��2������
Fe��OH��2��ˮ���ǰ�ɫ�������ڿ������ױ��������йص�ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______��ʵ���ҿ����������ַ����Ƶð�ɫ��Fe��OH��2������