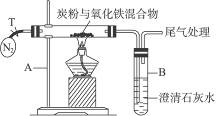
��Ŀ����
ʵ�����ﱣ�������Լ����䱣��ķ��������ɶ���ȷ���ǣ� ��
| ��� | �Լ� | ���淽�� | ���� |
| A | �������ƹ��� | ����ڴ������Ĺ��ƿ�� | ��ֹ������е������Ӵ��������Լ���ˮ������������̼������ |
| B | ̼���ƾ��� | ����ڸ����ܷ�Ĺ��ƿ�� | ��ֹ�绯�����ѳɷ�ĩ |
| C | Һ�� | ����ڴ�������ϸ��ƿ�У�����ˮ�� | ��ֹ�ӷ� |
| D | �������Ʒ�ĩ | ����ڴ����������Լ�ƿ�� | ��ֹ����ˮ�ֶ����� |
B
A���Ϊ�����к��е�SiO2�Ỻ���ط�����Ӧ��SiO2+2NaOH====Na2SiO3+H2O�����ɵ�Na2SiO3ʹƿ���벣��������һ��������������A��淽��������������������Ϊ�������Ʋ��ᱻ�����е�����������B�̼���ƾ���Ӧ�����ڸ����ܷ�Ĺ��ƿ�У��������ڣ�̼���ƾ���ͻᷢ���绯��ʧȥ�ᾧˮ����ɷ�ĩ��B��ı��淽�������ɾ�����C��������еijɷ�����������������ѧ��Ӧ�����Ա���ʱ������������ˮ��IJ�����ȷ��Ŀ���Ƿ�ֹҺ��ӷ���C��ı��淽������D�����Na2O2��ĩʱ��Ҫע���ֹ�Ӵ�ˮ�֣�2Na2O2+2H2O====4NaOH+O2������CO2��2Na2O2+2CO2====
2Na2CO3+O2�������ʣ�����Ӧ�����ڴ������Ĺ��ƿ�С�
2Na2CO3+O2�������ʣ�����Ӧ�����ڴ������Ĺ��ƿ�С�

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
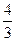 ��
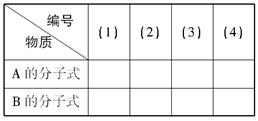
��
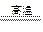 SiC+2CO����˵����ȷ���ǣ� ��
SiC+2CO����˵����ȷ���ǣ� ��