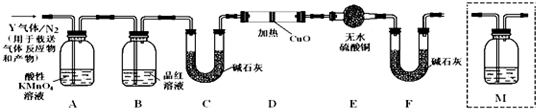
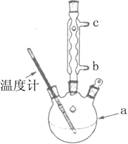
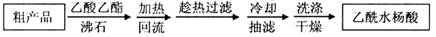��Ŀ����
ijѧϰС����ݻ�ѧ֪ʶ�����ϱ������ݣ�������±��е�ʵ�飬����֤���ԣ����̼����ӣ�̼������������в���������
���� | ���볣�����ܽ��(25��) |
C6H5OH | Ki =1.28��10-10 |
S = 9.3g/100gˮ | |
H2CO3 | Ki1 =4.3��10-7 |
Ki2 =5.6��10-11 |
��� | ���� | ����� | |
A | �����̼�� | ��̼��ƹ����еμ����� | �۲������������ |
B | ̼���뱽�� | �������Ʊ�����Һ�У�ͨ������CO2 | �۲���Һ�Ƿ����� |
C | ̼���뱽�� | ����ͬ���ʵ���Ũ�ȵ�̼������Һ�ͱ�������Һ | �Ƚ���Һ��pH |
D | HCO3���뱽�� | �ڱ�������Һ�У���������� Na2CO3��Һ | �۲�����Һ�Ƿ����� |
CD

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ