��Ŀ����
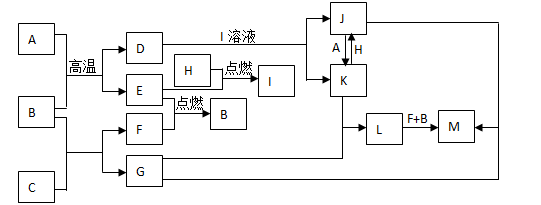
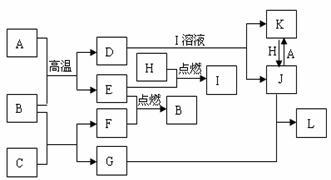
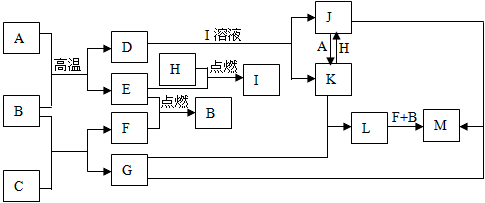
���п�ͼ���У���֪A��E��F��H�ǵ��ʣ�����ֻ��A�ǹ��壬�����������壬��H�dzʻ���ɫ��B�����Һ�壬G����ɫ��Ӧ�ʻ�ɫ��L�ǰ�ɫ������M�Ǻ��ɫ���������ַ�Ӧ��ijЩ����δ�����
�ش��������⣺
��1��д����ѧʽ��C________��I________��
��2��д�����ӷ���ʽ��
��D+I��Һ��____________________�� ��K+H��____________________��
��3��д����ѧ����ʽ����B+C��____________________�� ��L��M��____________________��
��4������J�е������ӵ���÷�����___________________________________��
��1��д����ѧʽ��C________��I________��
��2��д�����ӷ���ʽ��
��D+I��Һ��____________________�� ��K+H��____________________��
��3��д����ѧ����ʽ����B+C��____________________�� ��L��M��____________________��
��4������J�е������ӵ���÷�����___________________________________��
��1��Na2O2��HCl
��2����Fe3O4+8H+==Fe2++2Fe3++H2O����2Fe2++Cl2==2Fe3++2Cl-��
��3����2Na2O2+2H2O=4NaOH+O2������4Fe(OH)2+2O2+2H2O==4Fe(OH)3
��4������KSCN��Һ����Һ��Ѫ��ɫ��֤����Fe3+

��ϰ��ϵ�д�
�����Ŀ