��Ŀ����
����Ŀ��(1)ά����A�������ر��Ƕ��˵���������Ҫ���ã���ṹ��ʽ���£�

1 mol�û��������________mol Br2�����ӳɷ�Ӧ��
(2)���л���Ӧ�У���Ӧ����ͬ��������ͬ���ɵõ���ͬ���������ʽ��R���������������������ȥ��

(��ע��H��Br�ӳɵ�λ��)
��д��ʵ������ת��ĸ�����Ӧ�Ļ�ѧ����ʽ���ر�ע��д����Ӧ������
����CH3CH2CH2CH2Br������ת��ΪCH3CH2CHBrCH3��__________________��___________________��
����(CH3)2CHCH===CH2������ת��Ϊ(CH3)2CHCH2CH2OH��__________________��____________________��
���𰸡� 5 CH3CH2CH2CH2Br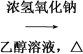 CH3CH2CH===CH2��HBr�� CH3CH2CH===CH2
CH3CH2CH===CH2��HBr�� CH3CH2CH===CH2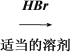 CH3CH2CHBr��CH3 (CH3)2CHCH===CH2
CH3CH2CHBr��CH3 (CH3)2CHCH===CH2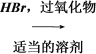 (CH3)2CHCH2CH2Br (CH3)2CHCH2CH2Br��H2O
(CH3)2CHCH2CH2Br (CH3)2CHCH2CH2Br��H2O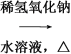 (CH3)2CHCH2CH2OH��HBr
(CH3)2CHCH2CH2OH��HBr
����������1��ά����A�к���5��̼̼˫����1 mol�û��������5mol Br2�����ӳɷ�Ӧ����2������CH3CH2CH2CH2Br������ת��ΪCH3CH2CHBrCH3�������Ϣ��֪��Ӧ�ȷ�����ȥ��Ӧ����1-��ϩ��Ȼ����HBr�����ӳɷ�Ӧ�������ķ�ӦΪCH3CH2CH2CH2Br![]() CH3CH2CH=CH2��CH3CH2CH=CH2HBr
CH3CH2CH=CH2��CH3CH2CH=CH2HBr![]() CH3CH2CHBrCH3�����ɣ�CH3��2CHCH=CH2������ת��Ϊ��CH3��2CHCH2CH2OH��-OH�ڶ�̼ԭ���ϣ�����HBr�ڹ����������������ɣ�CH3��2CHCH2CH2Br�����ڼ�����Һ��ˮ�⣬�����ķ�ӦΪ��CH3��2CHCH=CH2��HBr
CH3CH2CHBrCH3�����ɣ�CH3��2CHCH=CH2������ת��Ϊ��CH3��2CHCH2CH2OH��-OH�ڶ�̼ԭ���ϣ�����HBr�ڹ����������������ɣ�CH3��2CHCH2CH2Br�����ڼ�����Һ��ˮ�⣬�����ķ�ӦΪ��CH3��2CHCH=CH2��HBr![]() ��CH3��2CHCH2CH2Br����CH3��2CHCH2CH2Br
��CH3��2CHCH2CH2Br����CH3��2CHCH2CH2Br![]() ��CH3��2CHCH2CH2OH��
��CH3��2CHCH2CH2OH��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����֪�������ݣ�
���� | �۵�(��) | �е�(��) | �ܶ�(g��cm��3) |
�Ҵ� | ��117.0 | 78.0 | 0.79 |
���� | 16.6 | 117.9 | 1.05 |
�������� | ��83.6 | 77.5 | 0.90 |
ijѧ����ʵ������ȡ������������Ҫ�������£�

������2 mLŨ���ᡢ3 mL�Ҵ�(��18O)��2 mL����Ļ����Һ��
������ͼ���Ӻ�װ��(װ������������)��������Һ����С����ȼ���3 min��5 min��
�����Թ����ռ���һ���������ֹͣ���ȣ������Թ��Ҳ�������Ȼ���ô��ֲ㡣
�����������������ϴ�ӡ����
(1)��Ӧ��Ũ�����������______________________________________��
д����ȡ���������Ļ�ѧ����ʽ��_____________________________��
(2)����ʵ���б���̼������Һ��������________(����ĸ)��
A���к�������Ҵ� B���к����Ტ�����Ҵ�
C�����������������ܽ� D�������������ɣ���������
(3)����������ҪС����ȼ��ȣ�����Ҫ������___________________��
���������۲쵽��������_____________________________________��
�������Թ��е����ʷ����Եõ���������������ʹ�õ�������________������ʱ����������Ӧ������________(�����¿ڷ��������Ͽڵ���)����

