��Ŀ����
����Ŀ��������X��һ������,�ɲ�����ϩ��ױ�Ϊ��Ҫԭ��,������·�ߺϳ�:

��֪:RX![]() ROH;RCHO��CH3COOR��
ROH;RCHO��CH3COOR��![]() RCH=CHCOOR��
RCH=CHCOOR��
��ش�:
��1��B��D��F�Ļ�ѧ����ʽ_______________________________________��
��2��X�Ľṹ��ʽ_____��
��3�����ڻ�����X,����˵����ȷ����____��
A�����ܷ���ˮ�ⷴӦ B������Ũ���ᷢ��ȡ����Ӧ
C����ʹBr2/CCl4��Һ��ɫ D���ܷ���������Ӧ
��4�����л�����������F��ͬ���칹�����______��˫ѡ��
A��![]() B��
B��![]()
C��CH2CHCHCHCHCHCHCHCOOH D��![]()
���𰸡�CH3COOH��![]()
![]()
![]() ��H2O
��H2O ![]() C B��C
C B��C
��������
��ϩ��ˮ�����ӳɷ�Ӧ����A��AΪCH3CH2OH��A��������������B������B����ʽ֪��B�ṹ��ʽΪCH3COOH���ڹ��������¼ױ�����������ȡ����Ӧ����C�����ݷ�Ӧ����֪����ԭ��ȡ��������ԭ�ӣ���C����ʽ֪��C�ṹ��ʽΪ![]() ��C����ȡ����Ӧ����D��DΪ
��C����ȡ����Ӧ����D��DΪ![]() ��D������������Ӧ����EΪ
��D������������Ӧ����EΪ![]() ��B��D����������Ӧ����F��FΪ
��B��D����������Ӧ����F��FΪ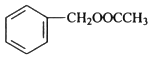 ��F������Ϣ�еķ�Ӧ����X������X����ʽ֪��X�ṹ��ʽΪ
��F������Ϣ�еķ�Ӧ����X������X����ʽ֪��X�ṹ��ʽΪ![]() ��
��
(1)BΪCH3COOH��DΪ![]() �����߷���������Ӧ����FΪ
�����߷���������Ӧ����FΪ ���÷�Ӧ����ʽΪCH3COOH+
���÷�Ӧ����ʽΪCH3COOH+![]()
![]()
 +H2O���ʴ�Ϊ��CH3COOH+
+H2O���ʴ�Ϊ��CH3COOH+![]()
![]()
 +H2O��
+H2O��
(2)ͨ�����Ϸ���֪��X�ṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)XΪ![]() ��X�к���̼̼˫��������������������ϩ���������������ʡ�A�������ܷ���ˮ�ⷴӦ��������ʹ����ʴ���B�����б�������������Ũ���ᷢ��ȡ����Ӧ���ʴ���C������̼̼˫���������ܺ��巢���ӳɷ�Ӧ��ʹBr2/CCl4��Һ��ɫ������ȷ��D������ȩ�������Բ��ܷ���������Ӧ���ʴ���ѡC��
��X�к���̼̼˫��������������������ϩ���������������ʡ�A�������ܷ���ˮ�ⷴӦ��������ʹ����ʴ���B�����б�������������Ũ���ᷢ��ȡ����Ӧ���ʴ���C������̼̼˫���������ܺ��巢���ӳɷ�Ӧ��ʹBr2/CCl4��Һ��ɫ������ȷ��D������ȩ�������Բ��ܷ���������Ӧ���ʴ���ѡC��
(4)FΪ ������9��̼ԭ�ӣ�2����ԭ�ӣ������Ͷ�Ϊ5��A��
������9��̼ԭ�ӣ�2����ԭ�ӣ������Ͷ�Ϊ5��A��![]() ����10��̼ԭ�ӣ�����B��
����10��̼ԭ�ӣ�����B��![]() ������9��̼ԭ�ӣ�2����ԭ�ӣ������Ͷ�Ϊ5����FΪͬ���칹�壬��ȷ��C��CH2CHCHCHCHCHCHCHCOOH������9��̼ԭ�ӣ�2����ԭ�ӣ������Ͷ�Ϊ5����FΪͬ���칹�壬��ȷ��D��
������9��̼ԭ�ӣ�2����ԭ�ӣ������Ͷ�Ϊ5����FΪͬ���칹�壬��ȷ��C��CH2CHCHCHCHCHCHCHCOOH������9��̼ԭ�ӣ�2����ԭ�ӣ������Ͷ�Ϊ5����FΪͬ���칹�壬��ȷ��D��![]() ����10��̼ԭ�ӣ�����ѡBC��
����10��̼ԭ�ӣ�����ѡBC��

����Ŀ������������������Ⱦ�������أ�ԭ��֮һ�ǻ�����β���к���NO��NO2��CO�����壬�������糧�ͷų�������NOx��SO2��CO2������Ҳ����ԭ�����ڶ����е�һЩ���������һ�����о���
��1���� CH4����ԭ��������������������������Ⱦ��
��֪����CH4(g) + 4NO2(g) = 4NO(g) + CO2(g) + 2H2O(g) ��H = - 574 kJ/mol
��CH4(g) + 4NO(g) = 2N2(g) + CO2(g) + 2H2O(g) ��H = - 1160 kJ/mol
��H2O(g) = H2O(l) ��H = - 44.0 kJ/mol
д�� CH4(g)�� NO2(g)��Ӧ���� N2(g)��CO2(g)�� H2O(l)���Ȼ�ѧ����ʽ��____________��
��2������β���к���CO��NO2���ж����壬��������װβ������װ�ã���ʹ�ж�����ת��Ϊ�����塣4CO(g)��2NO2(g) ![]() 4CO2(g)��N2(g) ��H��-1200 kJ��mol-1,���ڸ÷�Ӧ���¶Ȳ�ͬ��T2��T1��������������ͬʱ������ͼ����ȷ����________������ţ���
4CO2(g)��N2(g) ��H��-1200 kJ��mol-1,���ڸ÷�Ӧ���¶Ȳ�ͬ��T2��T1��������������ͬʱ������ͼ����ȷ����________������ţ���

��3���û���̿��ԭ��Ҳ���Դ����������ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO��������ӦC(s)+2NO(g) ![]() N2(g)+CO2(g) ��H= a kJ/mol
N2(g)+CO2(g) ��H= a kJ/mol
��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ���Ũ�����£�
ʱ��/min Ũ��/(mol/L) | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.0 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
����ͼ�����ݷ���T1��ʱ���÷�Ӧ��0-20min��ƽ����Ӧ����
��v��NO��= _________ ������÷�Ӧ��ƽ�ⳣ��K=___________________��
��30min��ֻ�ı�ijһ�����������ϱ��������жϸı������������ ____________������ĸ���ţ�����˫ѡ��
A��ͨ��һ������CO2 B��������ʵĴ��� C���ʵ���С���������
D��ͨ��һ������NO E������һ�����Ļ���̿
����30min�������¶���T2�����ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ2:1:1����ﵽ��ƽ��ʱNO��ת����_______������ߡ����͡�����a _____0���>����<������
����Ŀ���±���������֮��ͨ��һ����Ӧ������ʵ����ͼ��ʾת����ϵ����
ѡ�� | X | Y | Z |
A | Al2O3 | NaAlO2 | Al(OH)3 |
B | SiO2 | Na2SiO3 | H2SiO3 |
C | CO2 | Na2CO3 | NaHCO3 |
D | NH3 | NO2 | HNO3 |

A. A B. B C. C D. D
����Ŀ�����ֶ�����Ԫ��x��y��z��d��e��f��g��hԭ���������ε�������ԭ�Ӱ뾶��������ۻ���������±���ʾ��
x | y | z | d | e | f | g | h | |
ԭ�Ӱ뾶/nm | 0.037 | 0.077 | 0.075 | 0.074 | 0.186 | 0.143 | 0.102 | 0.099 |
��������ϼۻ�������ϼ� | +1 | +4 | +5 | -2 | +1 | +3 | -2 | -1 |
�ش��������⣺
��1���Ƚ�d��f�������ӵİ뾶��С���û�ѧʽ��ʾ����ͬ����_______>________���Ƚ�g��h������������Ӧˮ���������ǿ����________>__________��
��2����x��d����Ԫ�����18���ӵķ��ӣ������ʽΪ____________�����ⶨ��Ϊ��Ԫ���ᣬ�����Ա�̼������д�����һ������ĵ��뷽��ʽ____________��
��3��y��d��e�������e2yd3��ˮ��Һ�ʼ��ԣ���ԭ����____________________�������ӷ���ʽ��ʾ������������e2yd3����Һ��ԭ����ͼ��ʾ��

���У����ӽ���Ĥʹ�õ���__________��������ӽ���Ĥ���������ӽ���Ĥ��������������������A�Ļ�ѧʽΪ____________��
��4��f�ķ�ĩ��1000��ʱ����N2��Ӧ�Ʊ�fN����f�ķ�ĩ����������NH4Cl���岢��ֻ�ϣ�������fN���Ʊ�������Ҫԭ����____________________________________��