��Ŀ����
8������ʵ�����������ʵ����۾���ȷ���ǣ�������| ѡ�� | ʵ����������� | ʵ����� |
| A | ��ij��ɫ���������ɫ��Ӧʵ�飬����ʻ�ɫ | ������һ�������� |
| B | ��SO2�ֱ�ͨ����ˮ�����Ը��������Һ��Ʒ����Һ�У���Һ����ɫ | ����֤��SO2����Ư���� |
| C | ��ij��Һ�еμ�ŨNaOH��Һ�����ȣ���ʪ���ɫʯ����ֽ�����Թܿڣ���ֽ���� | ����Һ�д���NH4+ |
| D | ������Һ�м�������ϡ������ȣ�Ȼ��ֱ�Ӽ�������Һ�ټ��ȣ����������� | ֤������δˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A����Ԫ�ص���ɫ��ӦΪ��ɫ����ij��ɫ���������ɫ��Ӧʵ�飬����ʻ�ɫ�������ʿ��������Ρ���Ƶ�������ƵĹ��������������ʣ�
B������������л�ԭ�ԣ��ܱ�ǿ����������������������ֻ�ԭ�ԣ�
C��笠����Ӻ�NaOH��Ӧ����һˮ�ϰ���һˮ�ϰ��ڼ��������·ֽ����ɰ�����������ʹʪ���ɫʯ����ֽ����ɫ��
D��������Ӧ�����ڼ��������½��У�
��� �⣺A����Ԫ�ص���ɫ��ӦΪ��ɫ����ij��ɫ���������ɫ��Ӧʵ�飬����ʻ�ɫ�������ʿ��������Ρ�NaOH�������ơ��������ơ�Na�ȣ���A����
B������������л�ԭ�ԣ��ܱ�ǿ����������������������ֻ�ԭ�ԣ�������Ը��������Һ������ǿ�����ԣ��ܽ����������������������ʹ�����������ֻ�ԭ�Զ�����Ư���ԣ���B����
C��笠����Ӻ�NaOH��Ӧ����һˮ�ϰ���һˮ�ϰ��ڼ��������·ֽ����ɰ�����������ʹʪ���ɫʯ����ֽ����ɫ�����Ը�ʵ����֤����Һ�к���笠����ӣ���C��ȷ��
D��������Ӧ�����ڼ��������½��У������ڵμ�������Һ֮ǰӦ�ü���NaOH��Һ���к�δ��Ӧ��ϡ���ᣬ����ʵ�鲻�ɹ�����D����
��ѡC��
���� ���⿼�黯ѧʵ�鷽�����ۣ�Ϊ��Ƶ���㣬�漰ʵ��������������ʡ�Ԫ�ؼ��顢���Ӽ����֪ʶ�㣬��ȷʵ��ԭ�������������ǽⱾ��ؼ���ע���ʵ�������Լ�ʵ��ԭ�������жϣ��״�ѡ����D��

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д���1��NԪ��λ�����ڱ��ڶ����ڣ��ڢ�A�壬CԪ�ص�һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����146C��
��2���á���������������=����գ�
| ���Ӱ뾶 | �õ������� | ���� | ������ |
| O2-��Al3+ | 16O=18O | H2CO3��HNO3 | Fe��Al |
��4��ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ
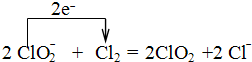 ��
����5������Ƭ�������ȥ����Ĥ����̼���õ������Ӻ����ϡNaOH��Һ�п��Թ���ԭ��أ��������Ϊ̼������ظ�����Ӧ�ĵ缫����ʽΪAl-3e-+4OH-=AlO2-+2H2O��
| ��������Ʊ������� | ���ᡢ�Ҵ���Ũ�����Ʊ��������� | ������������������Һ���� | �Ʊ�TNT |
| �ӳɷ�Ӧ | ȡ����Ӧ | ��ȥ��Ӧ | ������Ӧ |
| A | B | C | D |
| A�� | A | B�� | B | C�� | C | D�� | D |
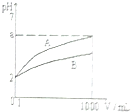 pH=2��A��B��������Һ��1mL���ֱ��ˮϡ�͵�1000mL������Һ��pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
pH=2��A��B��������Һ��1mL���ֱ��ˮϡ�͵�1000mL������Һ��pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | A��B��������Һ���ʵ���Ũ����� | B�� | ϡ�ͺ�A����Һ�����Ա�B����Һ�� | ||
| C�� | ��A=5ʱ��A��ǿ�ᣬB������ | D�� | ��5��a��2����A��B�������� |
| A�� | �ۻ�ʱ������ | |
| B�� | �������ӻ�������Ǽ��Թ��ۻ����� | |
| C�� | ˮ��Һ�ĵ��������ܲ� | |
| D�� | ��Һ�����ʷ��Ӻ����ʵ���������ӹ��� |
| A�� | ���� | B�� | �Ȼ�ͭ��Һ | C�� | Cl2 | D�� | O2 |

 ��
��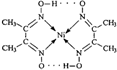 ������3d���ӵ�Ӱ�죬Ԫ�����ڱ��е������ڹ���Ԫ�ص������������γɶ��ֶ���������
������3d���ӵ�Ӱ�죬Ԫ�����ڱ��е������ڹ���Ԫ�ص������������γɶ��ֶ���������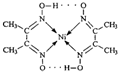 �����ڵ�������û��B �����ţ���
�����ڵ�������û��B �����ţ���