��Ŀ����
(12��)
(1)�Լ��顢����������������ҺΪԭ�ϣ�ʯīΪ�缫���Թ���ȼ�ϵ�ء��õ�صĸ�����ӦʽΪ ��
(2)����ͼ��ʾװ�ý��е�����A��B��Ϊʯī�缫��CΪ����CuS04��Һ��������ܷ�Ӧ����ʽΪ ��

���һ��ʱ���ȡ���缫������Һ�м��������� (�ѧʽ)��ʹC��Һ�ָ������ǰ�ijɷֺ�Ũ�ȡ�
����A��B��Ϊ���缫��CΪNa2SO4��Һ(����̪)�����һ��ʱ��� (�A����B��)�������Ժ�ɫ�����缫ȡ��������ʹ��Һ��Ͼ��ȣ������Һ��pH 7(�<������=����>��)��
����A��BΪͬһ���ϵĵ缫��CΪCuCl2��Һ����������CuCl2��Һ��Ũ��ʼ�� ���ֲ��䣬��A��BΪ (�ѧʽ)�缫������·����0��04 mol����ͨ��ʱ����������( ) g��
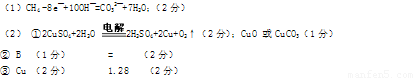
����������

(12��)��1�����϶�һ�š����³ɹ���ʵ�����й��ˡ����¡������룮���϶�һ�š�ʹ�õ��ƽ�����Һ���Һ���������ƽ������ŵ�����ͬ����ʱ�������ų��������࣬����Ϊˮ����Ⱦ��
��֪��H2(g)��O2(g)=H2O(l)����H=��285.8 kJ/mol ; H2(g)=H2(l)����H=��0.92 kJ/mol
O2(g)=O2(l)����H����6.84 kJ/mol ; H2O(l)=H2O(g)����H����44.0 kJ/mol
��д��Һ���Һ��������̬ˮ���Ȼ�ѧ����ʽ��_______________________________.
��2����֪����NH3(g)��HCl(g)===NH4Cl(s) ��H����176 kJ/mol
��NH3(g)��H2O(l)===NH3��H2O(aq) ��H ����35.1 kJ/mol
��HCl(g)===HCl(aq)�� ��H �� ��72.3 kJ/mol
��NH3��H2O(aq)��HCl(aq)===NH4Cl(aq)��H2O(l) ��H����52.3 kJ/mol
��NH4Cl(s)===NH4Cl(aq)�Ħ�H��______
��3���ֱ�ȡ40 mL��0.50 mol/L������0.55 mol/L����������Һ�����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ���ش��������⣮
�� ������ϡǿ�ᡢϡǿ�Ӧ����1 molˮʱ�ų�57.3 kJ��������д����ʾϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ __
�� �������������������Һ���ܶȶ���1 g/cm3����֪�кͺ�������Һ�ı�����
c��4.18 J/(g����)��Ϊ�˼����к��ȣ�ʵ��ʱ���������������(�����)__________��
A����Ӧǰ������Һ���¶� B����Ӧǰ������Һ������
C����Ӧǰ����������Һ���¶� D����Ӧǰ����������Һ������
E����Ӧ������Һ������¶� F����Ӧ������Һ������
�� ijѧ��ʵ���¼�������£�
|
ʵ�� ��� |
��ʼ�¶�t1/�� |
��ֹ�¶�t2/�� |
|
|
���� |
�������� |
�����Һ |
|
|
1 |
20.0 |
20.1 |
23.2 |
|
2 |
20.2 |
20.4 |
23.4 |
|
3 |
20.5 |
20.6 |
23.6 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к��Ȧ�H��__________ __��
�ܼٶ���ѧ���IJ�����ȫͬ�ϣ�ʵ���и���100 mL 0.5 mol/L�����100 mL 0.55 mol/L����������Һ���з�Ӧ��������ʵ����ȣ����ų�������________(���ȡ�����ȡ�)�������к���__________(���ȡ�����ȡ�)��
 �����ĺ�������������Ϊ �� ��
�����ĺ�������������Ϊ �� �� 