��Ŀ����
��20�֣����ܻ�����ı�����Һ�����ܽ�ƽ��,����:AgCl(s) Ag++Cl-,Ag2CrO4(s)
Ag++Cl-,Ag2CrO4(s) 2Ag++
2Ag++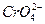 ����һ���¶���,��֪:Ksp(AgCl)=c(Ag+)��c(Cl-)=1.8��10-10,Ksp(Ag2CrO4)=��c(Ag+)��2��c(
����һ���¶���,��֪:Ksp(AgCl)=c(Ag+)��c(Cl-)=1.8��10-10,Ksp(Ag2CrO4)=��c(Ag+)��2��c(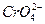 )=1.9��10-12,����0.01 mol��L-1 AgNO3��Һ�ζ�0.01 mol��L-1 KCl��0.001 mol��L-1 K2CrO4�����Һ,ͨ������ش�:
)=1.9��10-12,����0.01 mol��L-1 AgNO3��Һ�ζ�0.01 mol��L-1 KCl��0.001 mol��L-1 K2CrO4�����Һ,ͨ������ش�:
(1)Cl-��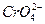 ˭�ȳ���?
˭�ȳ���?
(2)���ճ���Ag2CrO4����ʱ,��Һ��Cl-�����ʵ���Ũ���Ƕ���?(������Һ�ڷ�Ӧ���������)
 Ag++Cl-,Ag2CrO4(s)
Ag++Cl-,Ag2CrO4(s) 2Ag++
2Ag++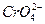 ����һ���¶���,��֪:Ksp(AgCl)=c(Ag+)��c(Cl-)=1.8��10-10,Ksp(Ag2CrO4)=��c(Ag+)��2��c(
����һ���¶���,��֪:Ksp(AgCl)=c(Ag+)��c(Cl-)=1.8��10-10,Ksp(Ag2CrO4)=��c(Ag+)��2��c(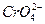 )=1.9��10-12,����0.01 mol��L-1 AgNO3��Һ�ζ�0.01 mol��L-1 KCl��0.001 mol��L-1 K2CrO4�����Һ,ͨ������ش�:
)=1.9��10-12,����0.01 mol��L-1 AgNO3��Һ�ζ�0.01 mol��L-1 KCl��0.001 mol��L-1 K2CrO4�����Һ,ͨ������ش�:(1)Cl-��
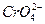 ˭�ȳ���?
˭�ȳ���?(2)���ճ���Ag2CrO4����ʱ,��Һ��Cl-�����ʵ���Ũ���Ƕ���?(������Һ�ڷ�Ӧ���������)
(1)Cl-�ȳ��� (2)4.13��10-6 mol��L-1
(1)�����������ʵ�Ksp,������ɸ��Եij��������c(Ag+),�Ƚ�����c(Ag+),˭��c(Ag+)С˭�ȳ�����
����Cl-: ��
��
����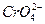 :
: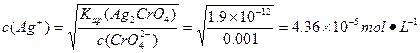 ��
��
��������AgCl������
(2)���c(Ag+)��2��c(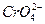 )=1.9��10-12,��c(Ag+)��2��0.001 mol��L-1=1.9��10-12,c(Ag+)=4.36��10-5 mol��L-1,c(Ag+)��c(Cl-)=1.8��10-10,4.36��10-5��c(Cl-)=1.8��10-10,c(Cl-)=4.13��10-6 mol��L-1��
)=1.9��10-12,��c(Ag+)��2��0.001 mol��L-1=1.9��10-12,c(Ag+)=4.36��10-5 mol��L-1,c(Ag+)��c(Cl-)=1.8��10-10,4.36��10-5��c(Cl-)=1.8��10-10,c(Cl-)=4.13��10-6 mol��L-1��
����Cl-:
 ��
������
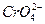 :
: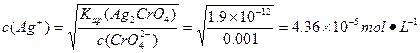 ��
����������AgCl������
(2)���c(Ag+)��2��c(
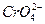 )=1.9��10-12,��c(Ag+)��2��0.001 mol��L-1=1.9��10-12,c(Ag+)=4.36��10-5 mol��L-1,c(Ag+)��c(Cl-)=1.8��10-10,4.36��10-5��c(Cl-)=1.8��10-10,c(Cl-)=4.13��10-6 mol��L-1��
)=1.9��10-12,��c(Ag+)��2��0.001 mol��L-1=1.9��10-12,c(Ag+)=4.36��10-5 mol��L-1,c(Ag+)��c(Cl-)=1.8��10-10,4.36��10-5��c(Cl-)=1.8��10-10,c(Cl-)=4.13��10-6 mol��L-1��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
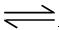 Ag+(aq)+ Cl��(aq) ��25��ʱ���Ȼ�����Ksp = 1.8��10��10
Ag+(aq)+ Cl��(aq) ��25��ʱ���Ȼ�����Ksp = 1.8��10��10 Ca2++2OH-
Ca2++2OH-