��Ŀ����
5����4mol A�����2mol B������2L�Ķ����ܱ������л�ϣ�����һ�������·������·�Ӧ��2A��g��+B��g��?2C��g������2s����C�����ʵ���Ũ��Ϊ0.6mol•L-1���������м���˵������������A��ʾ�����ʱ���ƽ������Ϊ0.3mol•L-1•s-1
��������B��ʾ�����ʱ���ƽ������Ϊ0.6mol•L-1•s-1
��2sʱ����A��ת����Ϊ30%
��2sʱ����B�����ʵ���Ũ��Ϊ0.3mol•L-1
������ȷ���ǣ�������
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ۢ� |
���� �ٸ���v=$\frac{��c}{��t}$����v��C�����ٸ�������֮�ȵ��ڻ�ѧ������֮�ȼ���v��A����
����������֮�ȵ��ڻ�ѧ������֮�ȼ���v��B����
�۸���n=cV����C�����ʵ����仯�������ݷ���ʽ��֪��n��A��=��n��C����A��ת����=$\frac{�μӷ�ӦA�����ʵ���}{A����ʼ���ʵ���}$��100%��
��Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ��ݴ˼���B��Ũ�ȱ仯������������2SʱB��Ũ�ȣ�
��� �⣺��2s����C��Ũ��Ϊ0.6mol•L-1����v��C��=$\frac{0.6mol/L}{2s}$=0.3mol/��L��min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��A��=v��C��=0.3mol/��L��min�����ʢ���ȷ��
������֮�ȵ��ڻ�ѧ������֮�ȣ���v��B��=$\frac{1}{2}$v��C��=$\frac{1}{2}$��0.3mol/��L��min��=0.15mol/��L��min�����ʢڴ���
������C�����ʵ���Ϊ2L��0.6mol•L-1=1.2mol�����ݷ���ʽ��֪��n��A��=��n��C��=1.2mol����A��ת����=$\frac{1.2mol}{4mol}$��100%=30%���ʢ���ȷ��
��Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ���B��Ũ�ȱ仯��=$\frac{1}{2}$��0.6mol•L-1=0.3mol/L����2sʱB��Ũ��Ϊ$\frac{2mol}{2L}$-0.3mol/L=0.7mol/L���ʢܴ���
��ѡA��
���� ���⿼�黯ѧƽ���뻯ѧ��Ӧ���ʵ��йؼ��㣬�ѶȲ����ضԻ���֪ʶ�Ĺ��̣�ע�ⷴӦ����ͨ�����ö��巨�����ʹ��ɼ��㣮

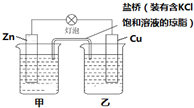
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
����˵������ȷ���ǣ�������
| A�� | ������ʱ��M��������N�ĵ� | B�� | M��N��Ϊͬ���칹�� | ||
| C�� | N��һ�ȴ���ֻ��һ�� | D�� | �����¶�������Nת����M |
| A�� | ��֪��HI��g��?$\frac{1}{2}$H2��g��+$\frac{1}{2}$I2��s����H=-26.5kJ/mol���ɴ˿�֪1mol HI�������ܱ������г�ַֽ����Էų�26.5kJ������ | |
| B�� | ��֪��2H2��g��+O2��g��=2H2O��l����H=-571.6 kJ/mol����������ȼ����Ϊ��H=-285.8 kJ/mol | |
| C�� | �£�N2H4����һ�����ڻ����ȼ�ϵ�ص�ԭ�ϣ���֪ 2H2O��g��+O2��g��=2H2O2��l����H=+108.3kJ/mol �� N2H4��l��+O2��g��=N2��g��+2H2O��g����H=-534.0kJ/mol �� ���з�Ӧ��N2H4��l��+2H2O2��l��=N2��g��+4H2O��l����H=-642.3kJ/mol | |
| D�� | ��20.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7 kJ����������ϡ�����ϡNaOH��Һ��Ӧ���Ȼ�ѧ����ʽΪ�� NaOH��aq��+CH3COOH��aq��=CH3COONa��aq��+H2O��l����H=-57.4 kJ/mol |
| A�� | 4 | B�� | 3 | C�� | 2 | D�� | 1 |
| A�� | BC����������������ɢϵ�ı��������Ƕ�������� | |
| B�� | ��С�ձ���25mL����ˮ�������ڣ����ˮ����μ���5��6���Ȼ���������Һ�������������Һ�ʺ��ɫ������ֹͣ���ȣ�����ȡFe��OH��3���� | |
| C�� | Fe��OH��3���������ڵ糡Ӱ���½��������˶���˵��Fe��OH��3��������� | |
| D�� | ��Fe��OH��3��������μ���ϡH2SO4��Һʱ����ʼʱ��������ۣ��ټ�����μ�ʱ�������ֻ���ʧ |
| A�� | һ�������£�����Ӧ�������ӿ컯ѧ��Ӧ���� | |
| B�� | ����ѹǿ���϶���ӿ컯ѧ��Ӧ���� | |
| C�� | ������������Ļ�ѧ������ԽС�������ʵ�������Խ�ͣ�����Խ�ȶ� | |
| D�� | �����¶���ʹ��λ����ڵĻ���������ӣ��Ӷ�����ѧ��Ӧ���� |