��Ŀ����
�� 6 �֣��ش��������⣺
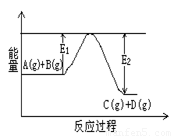
��1����ӦA(g)+B(g)![]() C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)=CO (g)+ H2O (g)�� ��H1= + 34.0 kJ/mol
����(g)=CO2 (g)+ H2(g) ��H2=��7.0 kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2 ����̬H2������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ��H2O2����
����0.4molҺ̬�º�0.8molҺ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ
������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ
��
��6�֣� (1) < ��1�֣�
(2) CH2O2 ��1�֣� CO2��g��+ H2��g��= CO��g��+ H2O��g����H= +41.0 kJ��mol��1��2�֣�
(3) N2H4��l��+ 2 H2O2��l��= N2��g��+ 4 H2O��g����H= --641.75 kJ��mol��1��2�֣�
����:��

 ����ѧ����ϵ�д�
����ѧ����ϵ�д� C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������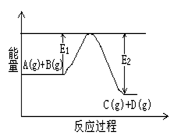
 O���Ȼ�ѧ����ʽΪ ��
O���Ȼ�ѧ����ʽΪ �� ����Һ̬˫��ˮ��H2O2����
����Һ̬˫��ˮ��H2O2���� C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������