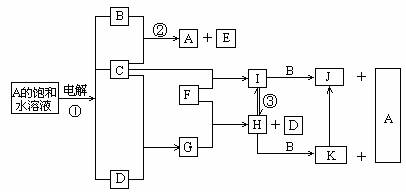
��Ŀ����
��ͼ�Dz���Ԫ�صĵ��ʼ��仯�����ת����ϵͼ(�йط�Ӧ����������ȥ)����֪��B��C��G��L ��Ϊ���ʣ������ڳ��¡���ѹ�£�G�ǹ��壬B��C��L�����壻������E����Ư���ԣ�����ɫ��Ӧ�ʻ�ɫ��F �ڳ�������Һ�壻K��M ��Ϊ������ˮ�ij���������K Ϊ���ɫ��

��ش��������⣺
(l)D�ĵ���ʽ��____________________________��
(2)��Ӧ�ٵ����ӷ���ʽ��____________________________��
(3)��Ӧ�ڵ����ӷ���ʽ��____________________________��
(4)��Ӧ�۵Ļ�ѧ����ʽ��___________________________________��
(5)����J��Һʱ�������___________________________________��
(6)ʵ������J�Ʊ�����M �ķ������ý�ͷ�ι���ȡA��Һ�����ιܼ�˲���ʢ������J��Һ�Թܵײ�����������A ��Һ���ɣ��۲쵽������Ϊ��____________________________������������������_____________________��
�� ![]() ��1�֣�
��1�֣�
(2) Cl2 +2OH- ===ClO- +C1-+H2O ��2�֣�
(3) Fe +2Fe3+ ===3Fe2+ (2��)
(4) 4Fe��OH��2 +O2 +2H2O=== 4Fe��OH��3����2�֣�
(5) ϡHC1 ��м��2 ��)
(6) ��ʼ����һ�ְ�ɫ����״������Ȼ��Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ�� �������ɵ�Fe(OH)2�����Ӵ�O2��3 ��)

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| |||||||||||||||||||||||
| |||||||||||||||||||||||