��Ŀ����
����8�֣� A��B��C��D��E�Ƕ�����Ԫ�أ����ڱ���A��B��B��C���ڣ�A��E������������֮��Ϊ2:3��B��������������E��������������1��������������D2C2��ˮ��Ӧ����C�ĵ��ʣ�����Һʹ��̪��Һ��졣
(1) E��Ԫ�ط�����__________��
(2)A��B��C���⻯���ȶ�����ǿ������˳��Ϊ(�÷���ʽ��ʾ)__________________��B���⻯���B������������ˮ���ﷴӦ����Z����Z�ľ�������Ϊ____________���õ���ʽ��ʾAE2���γɹ���________________________��
(3)��A��B��C��D��E�е�һ�ֻ���Ԫ����ɵ�����������Fe3+�����ӷ���ʽΪ__________________________��
(1) S�� (2) H2O��NH3��CH4��
���Ӿ��壻 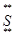 ��+
��+C
+��
![]()
![]() ��
��C
��
![]()
(3)Fe3++3SCN-= Fe��SCN��3��
����:����������D2C2��ˮ��Ӧ����C�ĵ��ʣ�����Һʹ��̪��Һ��죬��֪CΪ����DΪ�ơ����ڱ���A��B��B��C���ڣ�A��E������������֮��Ϊ2:3������֪��AΪ̼ BΪ�� CΪ�� EΪ�������������� KSCN���顣

��ϰ��ϵ�д�
�����Ŀ
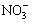 ��S
��S ��Cl����C
��Cl����C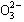 ��ijһ�֡�
��ijһ�֡�