��Ŀ����
��7�֣���һ��ɫ����Һ����֪���п��ܺ���Fe3+��Mg2+��Cu2+��Al3+��NH4+������һ�ֵ���ɫ��ĩ�����ȣ�������������������ʵ����뵭��ɫ��ĩ�����ʵ�����ϵ��ͼ��ʾ����ش�
��1������ɫ��ĩΪ_____________�������ƣ��������ʽΪ_____________
��2����Һ�п϶�û��_____________���ӡ�
��3����Һ�����ӵ����ʵ���֮��Ϊ____________________________��
��4���ٵ���ɫ��ĩ��ˮ��Ӧ�Ļ�ѧ����ʽΪ_______ ___________��
�ڳ������ּ���ʱ�����ӷ���ʽΪ_________________________________��
��1���������� ��1�֣�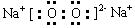 ��1�֣�
��1�֣�
��2��Fe3+��Cu2+ ��1�֣���3��n��Mg2+���Un��NH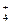 ���Un��Al3+��=1�U1�U1��2�֣�
���Un��Al3+��=1�U1�U1��2�֣�
��4����2Na2O2+2H2O�T4NaOH+O2����1�֣� ��Al��OH��3+OH���TAlO2��+2H2O��1�֣�
����

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
�����Ŀ



