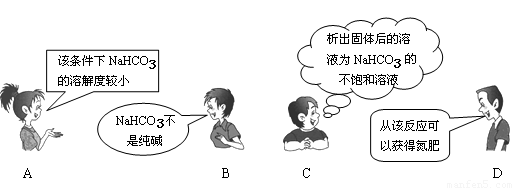
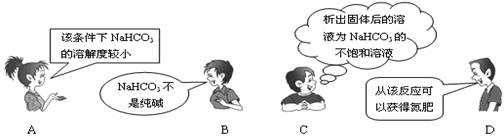
��Ŀ����
�����ġ������Ƽ����һ����Ӧ���Ͱ�����ˮ��ͨ�������̼���÷�Ӧ�ɱ�ʾΪNaCl+NH3+CO2+H2O=NaHCO3+NH4Cl�й����ʵ��ܽ���������£�g/100gˮ����
NaCl NaHCO3 NH4Cl
10��35.8 8.15 33.0
45��37.0 14.0 50.0
����45��ʱ����434g����ʳ��ˮ��ͨ��������������������ͨ�������̼��������Ӧ������ȫ���Լ��㲢�ش��������⣨������������λ��Ч���֣���
��1����Ӧ��ȫ��45��ʱ�����ľ���Ļ�ѧʽ��
��2�����˳�ȥ������ٽ�����10�棬��ʱ�����ľ����ǣ��ѧʽ��
NaCl NaHCO3 NH4Cl
10��35.8 8.15 33.0
45��37.0 14.0 50.0
����45��ʱ����434g����ʳ��ˮ��ͨ��������������������ͨ�������̼��������Ӧ������ȫ���Լ��㲢�ش��������⣨������������λ��Ч���֣���
��1����Ӧ��ȫ��45��ʱ�����ľ���Ļ�ѧʽ��
NaHCO3
NaHCO3
�������������������������2�����˳�ȥ������ٽ�����10�棬��ʱ�����ľ����ǣ��ѧʽ��
NH4Cl��NaHCO3
NH4Cl��NaHCO3
�������������������������������1��45��ʱ��434g����ʳ��ˮ�У�NaCl������Ϊ434g��
=117g��n��NaCl��=
=2mol�����ݷ�Ӧ�ķ���ʽ��������NaHCO3������������ܽ�ȼ������������������
��2�������ˮ����������ϸ����ʵ��ܽ���ж��������岢����������
| 37 |
| 137 |
| 117g |
| 58.5g/mol |
��2�������ˮ����������ϸ����ʵ��ܽ���ж��������岢����������
����⣺��1��45��ʱ��434g����ʳ��ˮ�У�NaCl������Ϊ434g��
=117g��n��NaCl��=
=2mol��
��NaCl+NH3+CO2+H2O=NaHCO3+NH4Cl
1 1
2mol 2mol
n��NaHCO3��=2mol��
m��NaHCO3��=2mol��84g/mol=168g��
��ʱ����Һ��ˮ������Ϊ434g��
-2��18g=280g��
�ܽ�NaHCO3������Ϊ
��14��39.0g��
��������������Ϊ168g-39g=129g��
������NaHCO3���������Ϊ129g��
��2���ɣ�1����֪ˮ������Ϊ280g������˳�ȥ������ٽ�����10�棬
�ܽ�NaHCO3������Ϊ8.15g��
=22.8g��
����NaHCO3������Ϊ39.0-22.8=16.2g��
���ɵ�NH4Cl����Ϊ2mol��53.5g/mol=107g��
��Һ�ܽ��NH4Cl����Ϊ33.0g��
=92.4g��
��������NH4Cl����Ϊ107g-92.4g=14.6g��
�������������ʵ�ΪNH4Cl��NaHCO3��������Ϊ16.2g+14.6g=30.8g��
�����������ʵ�ΪNH4Cl��NaHCO3��������Ϊ30.8g��
| 37 |
| 137 |
| 117g |
| 58.5g/mol |
��NaCl+NH3+CO2+H2O=NaHCO3+NH4Cl
1 1
2mol 2mol
n��NaHCO3��=2mol��
m��NaHCO3��=2mol��84g/mol=168g��
��ʱ����Һ��ˮ������Ϊ434g��
| 100 |
| 137 |
�ܽ�NaHCO3������Ϊ
| 280 |
| 100 |
��������������Ϊ168g-39g=129g��
������NaHCO3���������Ϊ129g��
��2���ɣ�1����֪ˮ������Ϊ280g������˳�ȥ������ٽ�����10�棬
�ܽ�NaHCO3������Ϊ8.15g��
| 280 |
| 100 |
����NaHCO3������Ϊ39.0-22.8=16.2g��
���ɵ�NH4Cl����Ϊ2mol��53.5g/mol=107g��
��Һ�ܽ��NH4Cl����Ϊ33.0g��
| 280 |
| 100 |
��������NH4Cl����Ϊ107g-92.4g=14.6g��
�������������ʵ�ΪNH4Cl��NaHCO3��������Ϊ16.2g+14.6g=30.8g��
�����������ʵ�ΪNH4Cl��NaHCO3��������Ϊ30.8g��
���������⿼�鴿����Ʊ��ͼ��㣬��Ŀ�ѶȽϴ�ע���й��ܽ�ȼ���ķ�����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ