��Ŀ����
������������ȫ��ȷ����
�ٳ��³�ѹ�£�1 mol��(��CH3)�����ĵ�����Ϊ10NA
��1mol��8��̼ԭ�ӵ�ij�����ӣ�����γ�8mol̼̼����
���ڱ�״���£�11.2 L NO��11.2 L O2��Ϻ����������Ϊ0.75NA
��16.9 g ����������BaO2����������������������Ϊ0.2NA
��1 mol C10H22�����й��ۼ�����Ϊ31 NA
��1 mol Cl2������Ӧʱ��ת�Ƶĵ�����һ����2NA
�������£�42.0g��ϩ�ͱ�ϩ�Ļ�������к��е�̼ԭ����Ϊ3NA
���״���£����һ������ͭ��Ũ���ᷴӦ������22.4 L�����壬��ԭ������ķ�����һ������NA
�;���ͭ��������������6.4gʱ����·��ת�Ƶ�������0.2NA
�γ��³�ѹ�£�2.24L���Ȼ�̼��������ԭ��������0.4NA
(11)��H2O2+Cl2=2HCl+O2��Ӧ�У�ÿ����32 g��������ת��4NA������
�ٳ��³�ѹ�£�1 mol��(��CH3)�����ĵ�����Ϊ10NA
��1mol��8��̼ԭ�ӵ�ij�����ӣ�����γ�8mol̼̼����
���ڱ�״���£�11.2 L NO��11.2 L O2��Ϻ����������Ϊ0.75NA
��16.9 g ����������BaO2����������������������Ϊ0.2NA
��1 mol C10H22�����й��ۼ�����Ϊ31 NA
��1 mol Cl2������Ӧʱ��ת�Ƶĵ�����һ����2NA
�������£�42.0g��ϩ�ͱ�ϩ�Ļ�������к��е�̼ԭ����Ϊ3NA
���״���£����һ������ͭ��Ũ���ᷴӦ������22.4 L�����壬��ԭ������ķ�����һ������NA
�;���ͭ��������������6.4gʱ����·��ת�Ƶ�������0.2NA
�γ��³�ѹ�£�2.24L���Ȼ�̼��������ԭ��������0.4NA
(11)��H2O2+Cl2=2HCl+O2��Ӧ�У�ÿ����32 g��������ת��4NA������
�ܢݢߢ�
��

��ϰ��ϵ�д�
�����Ŀ
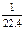 NA
NA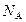 Ϊ�����ӵ�����������˵���в���ȷ���ǣ�����
Ϊ�����ӵ�����������˵���в���ȷ���ǣ�����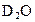 ����0.5mol
����0.5mol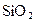 �����Ĺ��ۼ�����ĿΪ0.2
�����Ĺ��ۼ�����ĿΪ0.2

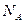 ��ʾ�����ӵ�������ֵ������˵����ȷ����
��ʾ�����ӵ�������ֵ������˵����ȷ����
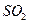 ���ܱ������к���
���ܱ������к���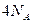 ��
��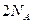 ������������������64g
������������������64g