��Ŀ����
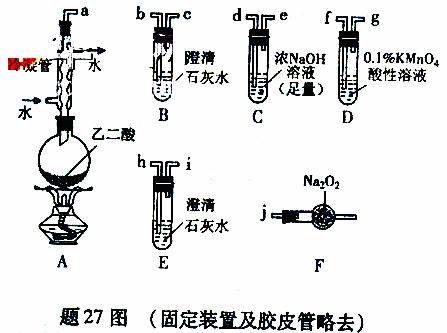
��15�֣�����ѧϰС������27ͼװ��̽���Ҷ��ᣨHOOC�DCOOH�����ȷֽ�IJ��ֲ��

��1�����飺
�ٰ��ӿ�˳��a�Db�Dc�Dd�De�Df�Dg�Dh����װ�ý���ʵ�顣B����Һ����ǣ�֤���ֽ������ ��װ��C�������� ��E����Һ����ǣ�D�е������� ��֤���ֽ������ ��
���Ҷ������ȷֽ�Ļ�ѧ����ʽΪ ��
��2�����飺
�ٽ��ӿ�a��j���ӽ���ʵ�飬�۲쵽F�����ɵ������ʹ�����ǵ�ľ����ȼ����F������Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��
�ڴ�A��F��ѡ��װ�ý���ʵ�飬֤������ͨ��D�������ܷ���Na2O2��Ӧ�����װ�ýӿ�����˳���� ��ʵ�����F�еĹ��������֤�ķ����� ������ѡ�Լ�����

������
��1����B�г���ʯ��ˮ����ǣ�֤���ֽ������CO2������װ��C�������dz�ֳ�ȥCO2����ֹ�Ժ��ʵ��������ĸ��ţ�E�г���ʯ��ˮ����ǣ�˵����CO2�������Ҷ��������CO2��Cװ�����Ѿ���NaOH������ȫ��CO2�IJ�����Դ��Dװ���и�����ض�CO��������D������Ϊ��Һ��ɫ�����dz����֤���ֽ������CO�����Ҷ������ȷֽ�Ļ�ѧ����ʽΪ��HOOC�DCOOH![]() CO2��+CO��+H2O����2���ٽӿ�a��j���ӽ���ʵ�飬�۲쵽F�����ɵ������ʹ�����ǵ�ľ����ȼ��˵��������������ͨ�������ܺ������Ҫ��CO2��CO2��Na2O2��Ӧ�Ļ�ѧ����ʽΪ��2CO2+Na2O2=== 2Na2CO3+O2������Ҫ�Ʊ����壬��������CO2���գ���ͨ�뵽Na2O2��֤��������˳��Ϊ��a��d��e��j��Ҫ֤�������˷�Ӧ����֤��F�е�������Na2CO3�������϶ࡣ
CO2��+CO��+H2O����2���ٽӿ�a��j���ӽ���ʵ�飬�۲쵽F�����ɵ������ʹ�����ǵ�ľ����ȼ��˵��������������ͨ�������ܺ������Ҫ��CO2��CO2��Na2O2��Ӧ�Ļ�ѧ����ʽΪ��2CO2+Na2O2=== 2Na2CO3+O2������Ҫ�Ʊ����壬��������CO2���գ���ͨ�뵽Na2O2��֤��������˳��Ϊ��a��d��e��j��Ҫ֤�������˷�Ӧ����֤��F�е�������Na2CO3�������϶ࡣ

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�