��Ŀ����
��ʵ������Ҫ����0.1 mol��L��1NaOH��Һ500mL��
��1�����ݼ�����������ƽ��ȡ������Ϊ__________g������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________(�����)����ͼ�����������⣬����������Һ����Ҫ�IJ��������� _____________��
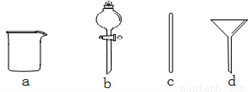
��2������ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ������ֻ��һ��)__________��
A��������ˮϴ���ձ�2�Ρ�3�Σ�ϴ��Һ��ע������ƿ����
B����ʢ��NaOH������ձ��м�������ˮ�ܽ�
C�����ձ�������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ����������µߵ���ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1 cm��2 cm��
��3����������������NaOH��ҺŨ��ƫ�ߵ��ǣ�_____��
A���ܽ����Һû����ȴ�����¾�ת��
B��ת��ʱû��ϴ���ձ���������
C��������ƿ��ˮ����ʱ�۾�����Һ��
D��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶��ߣ�
II����Ũ����ȡ������Ϊ100 mL �� A��B ����NaOH ��Һ�У��ֱ�ͨ��һ������ CO2 ������������Һ�еμ�0.1 mol/L ���� , ���� CO2 �����(��״��)����������������ϵ��ͼ��ʾ��
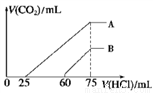
��4����A ���߱�����ԭ��Һͨ��CO2���������������ᷴӦ����CO2 ����������_________mL(��״��)��
��B ���߱�����ԭ��Һͨ��CO2��������Һ�����ʵĻ�ѧʽΪ______________��
III��ijѧ���� Na2CO3�� NaHCO3 ��ɵ�ij��������ʵ�飬�����������(��������ʵ���Ũ������Ҳ�����HCl�Ļӷ�)
ʵ����� | �� | �� | �� |
�������/mL | 50 | 50 | 50 |
��������/g | 4.11 | 8.22 | 16.44 |
�����������/L(���) | 1.008 | 2.016 | 2.016 |
��5��ԭ�������Ʒ�� n(Na2CO3)��n(NaHCO3)��____________ , ��������ʵ���Ũ��Ϊ________mol/L ��ʵ��۷�Ӧ���������������_____mL�ĸ�������Һ���ܰѻ����ȫ����Ӧ��



 ��������2-�һ�����
��������2-�һ����� N2O4������Ӧ���ȣ���������˵����ȷ����
N2O4������Ӧ���ȣ���������˵����ȷ����