��Ŀ����
����ͼ��ʾ���ʼ���ת���ϵ����Ӧ���ڹ�ҵ�Ͽ���������������C����Ӧ���ڹ�ҵ�Ͽ�����������J��Na2FeO4������Ӧ�١��ڡ��ܺ͢ݾ�����ˮ��Һ�н��еķ�Ӧ��������D��E��G�������壬B����ɫҺ�壻F��ˮ��Һ����Ϊɱ����������H��һ������ʯ����Ҫ�ɷ֣���������Ԫ����ɣ���������Ԫ�ص���������Ϊ70%��
��ش��������⣺
��д��F�Ļ�ѧʽ��________________��
�����ǽ���Ӧ���漰�Ļ�ѧ��ҵ��Ϊ ________________��
��д�����A��B���Һ��������Ӧʽ ��
�� д��B��D��Ӧ�����ӷ���ʽ________________________________________��
��д����Ӧ�ܵ����ӷ���ʽ______________________________________________��
��д����Ӧ�ݵ����ӷ���ʽ______________________________________________��
�˸������ƣ�Na2FeO4����������Ϊ��һ�֡���ɫ������Ч���ľ�ˮ������ԭ��Ϊ��
��Na2FeO4����ǿ�����Կ�ɱ��������
��___________________________________________________________________��
��NaClO���������������������������������������������� ��2�֣�
���ȼҵ������������������������������������������ ��2�֣�
��2Cl����2e��=Cl2 ��2�֣�
��Cl2�� H2O=== H+��Cl����HClO�� ��������������������������������2�֣�
��Fe2O3��6 H+===2Fe3����3H2O��������������������������������������2�֣�
��2Fe3����3ClO����10OH��===2FeO��3Cl����5H2O������������������ ��2�֣�
��Na2FeO4����ԭΪFe3����Fe3��ˮ������Fe(OH)3����������ˮ�е�������������γɳ�����ʹˮ���塡�������������������������������������� ��2�֣�
����:��

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д���12�֣��ڲ�ͬ�¶��£���ӦCO2(g)��H2(g) CO(g)��H2O(g)��ƽ�ⳣ��K���±���
CO(g)��H2O(g)��ƽ�ⳣ��K���±���
| �¶�/�� | 700 | 800 | 850 | 1000 | 1200 |
| ƽ�ⳣ��K | 2.6 | 1.7 | 1 | 0.9 | 0.6 |
��2��850��ʱ����������Ӧ��CO2��ת������ʱ��仯��ͼ��ʾ������������ͬʱ��������ͼ�л���700��ʱCO2��ת������ʱ��仯��ʾ��ͼ��ע����Ҫ�ı�ʾ����

��3����850��ʱ����������Ӧ�����±��е����ʵ���Ͷ����ݷ�Ӧ�����������������ƶ������� ������ţ���ƽ�����C�и����ʵİٷֺ�����ȵ�����__________(����� )
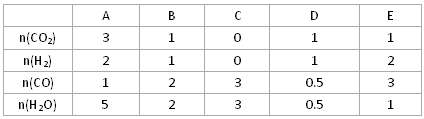
| | A | B | C | D | E |
| n(CO2)[ | 3 | 1 | 0 | 1 | 1 |
| n(H2) | 2 | 1 | 0 | 1 | 2 |
| n(CO) | 1 | 2 | 3 | 0.5 | 3 |
| n(H2O) | 5 | 2 | 3 | 0.5 | 1 |
��12�֣��ڲ�ͬ�¶��£���ӦCO2(g)��H2(g) CO(g)��H2O(g)��ƽ�ⳣ��K���±���
CO(g)��H2O(g)��ƽ�ⳣ��K���±���
|
�¶�/�� |
700 |
800 |
850 |
1000 |
1200 |
|
ƽ�ⳣ��K |
2.6 |
1.7 |
1 |
0.9 |
0.6 |
��1���÷�Ӧ�ġ�H 0�����>������=����<������������ƽ��������¶ȣ���CO2��ת���ʽ� ������Ӧ���� �����������С�����䡱����
��2��850��ʱ����������Ӧ��CO2��ת������ʱ��仯��ͼ��ʾ������������ͬʱ��������ͼ�л���700��ʱCO2��ת������ʱ��仯��ʾ��ͼ��ע����Ҫ�ı�ʾ����

��3����850��ʱ����������Ӧ�����±��е����ʵ���Ͷ����ݷ�Ӧ�����������������ƶ������� ������ţ���ƽ�����C�и����ʵİٷֺ�����ȵ�����__________(����� )
|
|
A |
B |
C |
D |
E |
|
n(CO2)[ |
3 |
1 |
0 |
1 |
1 |
|
n(H2) |
2 |
1 |
0 |
1 |
2 |
|
n(CO) |
1 |
2 |
3 |
0.5 |
3 |
|
n(H2O) |
5 |
2 |
3 |
0.5 |
1 |
(4) 850��ʱ����1L���ܱ������зֱ����2mol��CO2��H2�����㷴Ӧ����ƽ��ʱCO�����ʵ���Ũ�ȡ���д��������̣�
 CO(g)��H2O(g)��ƽ�ⳣ��K���±���
CO(g)��H2O(g)��ƽ�ⳣ��K���±���

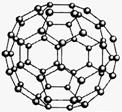 C60���ṹģ������ͼ��ʾ���ķ����ǻ�ѧ��Ĵ���֮һ��C60������ػ�������K3C60��K3C60���г����ԡ�k.s.5.u
C60���ṹģ������ͼ��ʾ���ķ����ǻ�ѧ��Ĵ���֮һ��C60������ػ�������K3C60��K3C60���г����ԡ�k.s.5.u