��Ŀ����
ʵ����Ҫ����0.5mol/L��NaOH��Һ100mL����
��1����Ҫ����NaOH������Ϊ______��
��2����������ƽ����NaOH����ʱ�������NaOH����______�����г�����
��3������ʱ����Ҫ�õ���������______������ĸ��
A���ձ����� B������̨�������У� C.100mL����ƿ D.50mL����ƿ E��������ƿ F����ͷ�ι� G��������
��4����ʵ���Ⱥ�˳����������ʵ�鲽��______�����ţ�
�ٽ������õ�NaOH���嵹���ձ��У�
�����ձ��е���Լ20mL����ˮ��
�۽��ܽⲢ��ȴ���NaOH��Һ��������ƿ��
��������ƿ�м�����ˮ��Һ����ƿ���̶�����1��2cm��
������������ˮϴ���ձ��Ͳ�����2��3�Σ�
��ϴ�Ӻ����ҺҲע������ƿ�У�
�߸Ǻ�ƿ�����������µߵ���ҡ�ȣ�
���ý�ͷ�ιܵμ�����ˮ��Һ����ƿ���̶������У�
��5���������ػ���������Ƶ�NaOH��Һ���ʵ���Ũ�ȹ�ƫ�͵���______
A��NaOH��Һδ����ȴ������ת��������ƿ��
B�����ݺ���Һ����ڿ̶��߲�������ˮ
C��������NaOH��Һ�������ձ���
D������ƿ��ԭ������������ˮ
��6��Ϊ���龫�δ��ȣ�������250mL 0.2mol/L NaCl�����Σ���Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ�����еĴ�����______��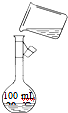
��1����Ҫ����NaOH������Ϊ______��
��2����������ƽ����NaOH����ʱ�������NaOH����______�����г�����
��3������ʱ����Ҫ�õ���������______������ĸ��
A���ձ����� B������̨�������У� C.100mL����ƿ D.50mL����ƿ E��������ƿ F����ͷ�ι� G��������
��4����ʵ���Ⱥ�˳����������ʵ�鲽��______�����ţ�
�ٽ������õ�NaOH���嵹���ձ��У�
�����ձ��е���Լ20mL����ˮ��
�۽��ܽⲢ��ȴ���NaOH��Һ��������ƿ��
��������ƿ�м�����ˮ��Һ����ƿ���̶�����1��2cm��
������������ˮϴ���ձ��Ͳ�����2��3�Σ�
��ϴ�Ӻ����ҺҲע������ƿ�У�
�߸Ǻ�ƿ�����������µߵ���ҡ�ȣ�
���ý�ͷ�ιܵμ�����ˮ��Һ����ƿ���̶������У�
��5���������ػ���������Ƶ�NaOH��Һ���ʵ���Ũ�ȹ�ƫ�͵���______
A��NaOH��Һδ����ȴ������ת��������ƿ��
B�����ݺ���Һ����ڿ̶��߲�������ˮ
C��������NaOH��Һ�������ձ���
D������ƿ��ԭ������������ˮ
��6��Ϊ���龫�δ��ȣ�������250mL 0.2mol/L NaCl�����Σ���Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ�����еĴ�����______��

��1������0.5mol/L��NaOH��Һ100mL�����������Ƶ�����Ϊm=0.5L��0.1mol?L-1��40g/mol=2.0g��
�ʴ�Ϊ��2.0g��
��2���������ƾ��и�ʴ�����׳��⣬Ӧ�����ձ���Ѹ�ٳ������ʴ�Ϊ���ձ���
��3�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������100mL����ƿ����ͷ�ιܡ�ҩ�ף�
��ѡ��ACFG��
��4���ɣ�3���в��������֪��ʵ���Ⱥ�˳��٢ڢۢݢޢܢ�ߣ��ʴ�Ϊ���٢ڢۢݢޢܢ�ߣ�
��5��A��Һ������������������ʣ����������ܽ���ȣ�δ��ȴ�����£����Ƚ���Һ��������ƿ���������Һ����ȴ��ᵼ����Һ���ƫС����ҺŨ��ƫ�ߣ���A�����ϣ�
B�����ݺ���Һ����ڿ̶��ߣ����Բ�������ˮ���̶��ߣ���������ҺŨ����Ӱ�죬��B�����ϣ�
C��������NaOH��Һ�������ձ��У���������ƿ���������Ƶ����ʵ�����С��������ҺŨ��ƫ�ͣ���C���ϣ�
D���������ˮ���ݣ�����ƿ��ԭ������������ˮ����������ҺŨ����Ӱ�죬��D�����ϣ�
��ѡC��
��6����ͬѧ������ƿ����Һ���ò�������������ֹ��Һ���䣬�ʴ�Ϊ��δ�ò�����������
�ʴ�Ϊ��2.0g��
��2���������ƾ��и�ʴ�����׳��⣬Ӧ�����ձ���Ѹ�ٳ������ʴ�Ϊ���ձ���
��3�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������100mL����ƿ����ͷ�ιܡ�ҩ�ף�
��ѡ��ACFG��
��4���ɣ�3���в��������֪��ʵ���Ⱥ�˳��٢ڢۢݢޢܢ�ߣ��ʴ�Ϊ���٢ڢۢݢޢܢ�ߣ�
��5��A��Һ������������������ʣ����������ܽ���ȣ�δ��ȴ�����£����Ƚ���Һ��������ƿ���������Һ����ȴ��ᵼ����Һ���ƫС����ҺŨ��ƫ�ߣ���A�����ϣ�
B�����ݺ���Һ����ڿ̶��ߣ����Բ�������ˮ���̶��ߣ���������ҺŨ����Ӱ�죬��B�����ϣ�
C��������NaOH��Һ�������ձ��У���������ƿ���������Ƶ����ʵ�����С��������ҺŨ��ƫ�ͣ���C���ϣ�
D���������ˮ���ݣ�����ƿ��ԭ������������ˮ����������ҺŨ����Ӱ�죬��D�����ϣ�
��ѡC��
��6����ͬѧ������ƿ����Һ���ò�������������ֹ��Һ���䣬�ʴ�Ϊ��δ�ò�����������

��ϰ��ϵ�д�
�����Ŀ
 ʵ����Ҫ����0.5mol/L��NaOH��Һ100mL����
ʵ����Ҫ����0.5mol/L��NaOH��Һ100mL����