��Ŀ����
Cu�Ļ��������������������Ҫ���ã���ͬ��Ӧ���Ƶò�ͬ״̬��Cu2O
��1����ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����
���ڼ�����������Һ̬�£�N2H4����ԭ����Cu��OH��2���Ʊ�����Cu2O��ͬʱ�ų�N2�����ռ���N2���Ϊ3.36L���ѻ���Ϊ��״����ʱ�����Ʊ�����Cu2O������Ϊ ��
��һ���¶��£���2 L�ܱ������м�������Cu2O��ͨ��0.20 molˮ������������Ӧ��
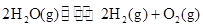
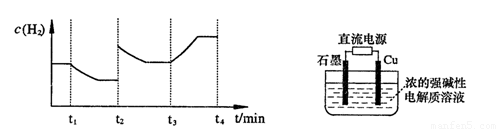
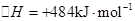 �����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ
��t2ʱ�ı����������Ϊ
������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ
��
�����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ
��t2ʱ�ı����������Ϊ
������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ
��
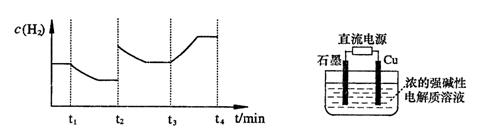
��2����֪��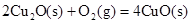 ��H����293kJ��mol��1
��H����293kJ��mol��1
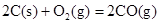 ��H����221kJ��mol��1
��H����221kJ��mol��1
��д��������̿�ۻ�ԭCuO��s���Ʊ�Cu2O��s�����Ȼ�ѧ����ʽ ��
��3���õ�ⷨҲ���Ʊ�Cu2O��ԭ��������ͼ��ʾ���������缫��Ӧ���Ա�ʾΪ ��
��1��Բ����ƿ��1�֣�
��2���رջ���A�ͷ�Һ©����������Բ����ƿ�����������Һ�����ߣ�˵�����������á���2�֣�
��3��3Cu��8H����2NO3��=3Cu2����2NO����4H2O��2�֣�
��4�������У�1�֣�����ΪNO����װ���е�������Ӧ������ˮ��ʹ��õ�NO�����������2�֣�
��5��B��1�֣���D��1�֣��� ��6����ʹ�����ܺ�����C����Һ����ƽ��2�֣��� ��7��27%����3�֣�
����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�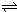 2H2��g��+O2��g����H=+484kJ?mol-1�����20minʱO2�����ʵ���Ϊ0.0016mol����ǰ20min�ķ�Ӧ����v��H2O��=
2H2��g��+O2��g����H=+484kJ?mol-1�����20minʱO2�����ʵ���Ϊ0.0016mol����ǰ20min�ķ�Ӧ����v��H2O��=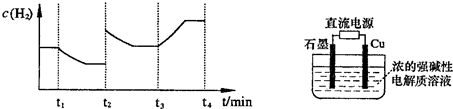
 �¶��£���2 L�ܱ������м�������Cu2O��ͨ��0.20 molˮ������������Ӧ��
�¶��£���2 L�ܱ������м�������Cu2O��ͨ��0.20 molˮ������������Ӧ��
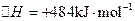 �����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ ��t2ʱ�ı����������Ϊ ������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ
�����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ ��t2ʱ�ı����������Ϊ ������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ �ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ ��
�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ ��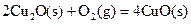 ��H����293kJ��mol��1
��H����293kJ��mol��1 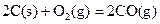 ��H����221kJ��mol��1
��H����221kJ��mol��1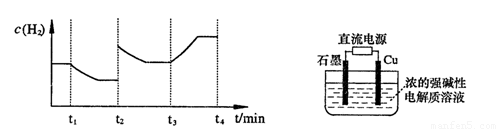
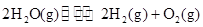
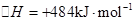 �����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ
��t2ʱ�ı����������Ϊ
������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ
��
�����20 minʱO2�����ʵ���Ϊ0.0016 mol����ǰ20 min�ķ�Ӧ����v��H2O��= �����¶��£���Ӧ��ƽ�ⳣ������ʽK= ����ͼ��ʾ��t1ʱ�̴ﵽƽ���ֻ�ı�һ�������ִﵽƽ��IJ�ͬʱ���ڣ�H2��Ũ����ʱ��仯���������t1ʱƽ����ƶ�����Ϊ
��t2ʱ�ı����������Ϊ
������K1��K2��K3�ֱ��ʾt1ʱ����ı�����������ʱ����ڵ�ƽ�ⳣ����t3ʱ��û�м���������ϵ�е��κ����ʣ���K1��K2��K3�Ĺ�ϵΪ
��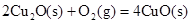 ��H����293kJ��mol��1
��H����293kJ��mol��1
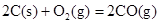 ��H����221kJ��mol��1
��H����221kJ��mol��1