��Ŀ����
��9�֣�����ʽ�ζ���ȷ��ȡ25��00mLijδ֪Ũ�ȵ���������һ�ྻ����ƿ�У�Ȼ����0��20mol��L -1������������Һ��ָʾ��Ϊ��̪�����ζ�������£�
|
|
NaOH��ʼ���� |
NaOH�յ���� |
|
��һ�� |
0��10mL |
18��50mL |
|
�ڶ��� |
0��20mL |
18��80mL |
��1�������������ݿ��Լ������������ʵ���Ũ��Ϊ mol��L-1��
��2���ﵽ�ζ��յ�ı�־��
��3�����²�����ɲⶨ���ƫ�ߵ�ԭ������� ��
A�����Ʊ���Һ�����������л���Na2CO3����
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
D���ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
E��δ�ñ�Һ��ϴ��ʽ�ζ���
��1��0��148��0��15mol/L ��
��2�� �����һ����Һ����ɫ��dz��ɫ������Ӳ���ɫ
��3��A�� D��E
��������
�����������1�����ݱ��������ݿ�֪�����������������ƽ��ֵΪ18.50 mL��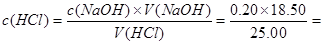 0.148mol/L��
0.148mol/L��
��2���ﵽ�ζ��յ�ı�־�ǵ����һ����Һ����ɫ��dz��ɫ������Ӳ���ɫ��
��3��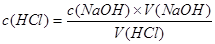 ����������Ƿ��ߣ���Ҫ�ǿ��������Ƶ������A�����Na2CO3���ʣ������������Ƶ����ƫ���ƫ�ߣ�B��ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ��������Ƶ����ƫС�����ƫ�ͣ�C����Ӱ�죻D��ζ��ܼ��촦����һ����Һ��˵���������Ƶ����ƫ���´���ҺŨ��ƫ��E��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ����±�ҺŨ��ƫС����Ҫ��������ʹ����ҺŨ�ȱ��
����������Ƿ��ߣ���Ҫ�ǿ��������Ƶ������A�����Na2CO3���ʣ������������Ƶ����ƫ���ƫ�ߣ�B��ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ��������Ƶ����ƫС�����ƫ�ͣ�C����Ӱ�죻D��ζ��ܼ��촦����һ����Һ��˵���������Ƶ����ƫ���´���ҺŨ��ƫ��E��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ����±�ҺŨ��ƫС����Ҫ��������ʹ����ҺŨ�ȱ��
���㣺��������к͵ζ�ԭ������������������
��������������Ҫ���DZ�Һ�����ƫ��ƫС�ȡ����ڽϼ��⡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���8�֣�����ʽ�ζ���ȷ��ȡ25.00mLijδ֪Ũ�ȵ�������Һ��һ�ྻ����ƿ�У�Ȼ����0.2000mol��L -1������������Һ��ָʾ��Ϊ��̪���ζ��ý�����£�
|
|
NaOH��ʼ���� |
NaOH�յ���� |
|
��һ�� |
0.40mL |
18.50mL |
|
�ڶ��� |
1.30mL |
18.05mL |
|
������ |
3.10mL |
21.20mL |
��1���ﵽ�ζ��յ��ʱ��Һ��ɫ�ı仯Ϊ ɫ�� ɫ��
��2�������������ݿ��Լ������������ʵ���Ũ��Ϊ ��
��3�����²�����ɲⶨ���ƫ�ߵ�ԭ������� ��
A�� �ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
B�� ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
C�� �ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
D�� δ�ñ�Һ��ϴ��ʽ�ζ���