��Ŀ����
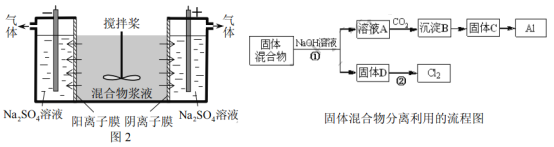
����Ŀ����14�֣�ij����ャҺ����Al(OH)3��MnO2������Na2CrO4,�����ǵ��������������ʹNa2CrO4������ȫ��ˮ������ij�о�С��������Ƶĵ�����װ�ã���ͼ2����ʹ��Һ����ɹ�������ͺ���Ԫ����Һ�����������á��ش��������е����⡣
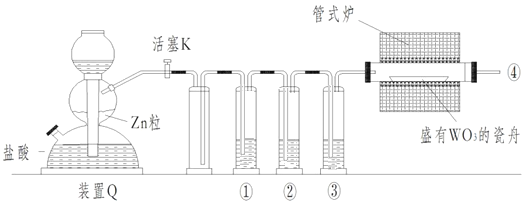
������������ķ�������ã�����ͼ�еIJ��ַ�������ͷ�Ӧ����δ������
��1����Ӧ�������Լ�NaOH�ĵ���ʽΪ_________��B��C�ķ�Ӧ����Ϊ__________��C��Al���Ʊ�������Ϊ______________��
��2����С��̽����Ӧ��������������D��Ũ�����ϣ������ȣ��ޱ仯��������Cl2���ɣ�����Ӧֹͣ������ʣ�࣬��ʱ�μ����ᣬ�ֲ���Cl2���ɴ��ж�Ӱ��÷�Ӧ��Ч���е������У�����ţ�___________��
a���¶� b��Cl-��Ũ�� c����Һ�����
��3��0.1 mol Cl2�뽹̿��TiO2��ȫ��Ӧ������һ�ֻ�ԭ�������һ����ˮ���TiO2��xH2O��Һ̬���������4.28 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
��������Ԫ����Һ�ķ��������
��4���ö��Ե缫���ʱ��![]() �ܴӽ�Һ�з��������ԭ����__________�������Ԫ�ص�������_________�����������ɵ�����Ϊ___________��д��ѧʽ����
�ܴӽ�Һ�з��������ԭ����__________�������Ԫ�ص�������_________�����������ɵ�����Ϊ___________��д��ѧʽ����
���𰸡���14�֣�
��1��![]() ���ȣ������գ� ��ⷨ
���ȣ������գ� ��ⷨ
��2��a c
��3��2Cl2(g)+ TiO2(s)+2C(s)===TiCl4(l)+2CO(g) ��H=85.6kJ��mol1
��4����ֱ���糡�����£�CrO42-ͨ�������ӽ���Ĥ���������ƶ������뽬Һ
CrO42-��Cr2O72- NaOH��H2
�����������Ե缫������ャҺʱ��Na+����������CrO42-����������Al(OH)3��MnO2�ڹ��������С������������NaOHʱ��Al(OH)3ת��ΪAlO2-��ͨ��CO2ת��ΪAl(OH)3�������ټ��ȷֽ�ΪAl2O3��������ڵ���Al��
��1��NaOH�ĵ���ʽΪ![]() ����������������B��C������Ϊ���Ȼ����գ�C��Al���Ʊ�������Ϊ��ⷨ��
����������������B��C������Ϊ���Ȼ����գ�C��Al���Ʊ�������Ϊ��ⷨ��
��2������ʵ�鷽����֪��Ӱ��÷�Ӧ���������¶Ⱥ���Һ����ȣ���ѡac��
��3���÷�Ӧ���Ȼ�ѧ����ʽΪ2Cl2(g)+ TiO2(s)+2C(s)===TiCl4(l)+2CO(g) ��H=85.6kJ��mol1��
��4���ö��Ե缫���ʱ��CrO42-�ܴӽ�Һ�з��������ԭ������ֱ���糡�����£�CrO42-ͨ�������ӽ���Ĥ���������ƶ������뽬Һ�������Ԫ�ص�������CrO42-��Cr2O72-�����������ɵ�����ΪNaOH��H2��