��Ŀ����
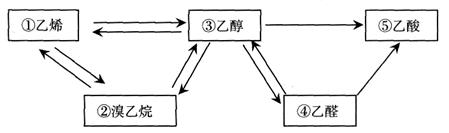
��12�֣���ͼ����ѧ�г������������ʼ���ת����ϵ��
��1����������������������� ������ţ���
��2���ܺ�̼�����Ʒ�Ӧ�������� ������ţ�����Ӧ�ķ���ʽΪ ��
��3��ʵ������ȡ��ϩ��ԭ���� ������ţ����÷�Ӧ�Ļ�ѧ����ʽΪ ����Ӧ������ ��
��4����ϩ���������ɾ���ϩ������ϩ�Ľṹ��ʽΪ �����Ƴɵ����Ͽ��Է����������ڼӹ������� ���ϣ�������ԡ����ȹ��ԡ�����
��5��д�������鷢����ȥ��Ӧ�ķ���ʽ ��

��1����������������������� ������ţ���
��2���ܺ�̼�����Ʒ�Ӧ�������� ������ţ�����Ӧ�ķ���ʽΪ ��
��3��ʵ������ȡ��ϩ��ԭ���� ������ţ����÷�Ӧ�Ļ�ѧ����ʽΪ ����Ӧ������ ��
��4����ϩ���������ɾ���ϩ������ϩ�Ľṹ��ʽΪ �����Ƴɵ����Ͽ��Է����������ڼӹ������� ���ϣ�������ԡ����ȹ��ԡ�����
��5��д�������鷢����ȥ��Ӧ�ķ���ʽ ��
����12�֣� ��1����
��2���� CH3COOH��NaHCO3��CH3COONa��H2O��CO2����2�֣�
��3���� CH3CH2OH CH2=CH2����H2O��2�֣��� ��ȥ��Ӧ
CH2=CH2����H2O��2�֣��� ��ȥ��Ӧ
��4��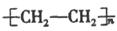 �� ������
�� ������
��5��CH3CH2Br��NaOH CH2=CH2����H2O��NaBr��2�֣�
CH2=CH2����H2O��NaBr��2�֣�
��2���� CH3COOH��NaHCO3��CH3COONa��H2O��CO2����2�֣�
��3���� CH3CH2OH
 CH2=CH2����H2O��2�֣��� ��ȥ��Ӧ
CH2=CH2����H2O��2�֣��� ��ȥ��Ӧ��4��
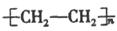 �� ������
�� ��������5��CH3CH2Br��NaOH
 CH2=CH2����H2O��NaBr��2�֣�
CH2=CH2����H2O��NaBr��2�֣���1����ϩֻ����̼������Ԫ�أ��������ࡣ
��2�������к����Ȼ����ܺ�̼�����Ʒ�Ӧ����CO2������ʽΪCH3COOH��NaHCO3��CH3COONa��H2O��CO2����
��3���Ҵ���Ũ����������·�����ȥ��Ӧ������ϩ���ݴ˿�����ȡ��ϩ������ʽΪ
CH3CH2OH CH2=CH2����H2O��
CH2=CH2����H2O��
��4����ϩ����̼̼˫�����ܷ����Ӿ۷�Ӧ���ɾ���ϩ����ṹ��ʽΪ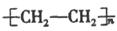 ��ֻ�����������ϲ��ܼ������ڼӹ������Ծ���ϩ�������������ϡ�
��ֻ�����������ϲ��ܼ������ڼӹ������Ծ���ϩ�������������ϡ�
��5�����������������ƵĴ���Һ�м��ȷ�����ȥ��Ӧ������ϩ������ʽΪCH3CH2Br��NaOH CH2=CH2����H2O��NaBr��
CH2=CH2����H2O��NaBr��
��2�������к����Ȼ����ܺ�̼�����Ʒ�Ӧ����CO2������ʽΪCH3COOH��NaHCO3��CH3COONa��H2O��CO2����
��3���Ҵ���Ũ����������·�����ȥ��Ӧ������ϩ���ݴ˿�����ȡ��ϩ������ʽΪ
CH3CH2OH
 CH2=CH2����H2O��
CH2=CH2����H2O����4����ϩ����̼̼˫�����ܷ����Ӿ۷�Ӧ���ɾ���ϩ����ṹ��ʽΪ
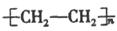 ��ֻ�����������ϲ��ܼ������ڼӹ������Ծ���ϩ�������������ϡ�
��ֻ�����������ϲ��ܼ������ڼӹ������Ծ���ϩ�������������ϡ���5�����������������ƵĴ���Һ�м��ȷ�����ȥ��Ӧ������ϩ������ʽΪCH3CH2Br��NaOH
 CH2=CH2����H2O��NaBr��
CH2=CH2����H2O��NaBr��
��ϰ��ϵ�д�
�����Ŀ
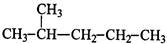 ��
��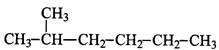
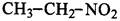 ��
��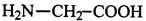
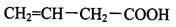 ��
��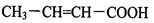
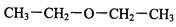 ��
��

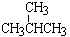 ��
��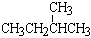 �� ��
�� ��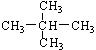 ��
��