��Ŀ����
����Ŀ����( N2H4)��һ�ָ���ȼ�ϣ��ڹ�ҵ��������;�㷺��
(1)0.5mol���к���_________________mol���Թ��ۼ���
(2)��ҵ�Ͽ����£�N2H4��������Cu(OH)2��Ӧ�Ʊ�����Cu2O��ͬʱ�ų�N2�÷�Ӧ�Ļ�ѧ����ʽΪ___________________��
(3)������ʱ����Ϊȼ�ϣ�˫��ˮΪ�����������߷�Ӧ�ɵ�����ˮ��������֪1.6gҺ̬����������Ӧ�зų�64.22kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ____________________________��
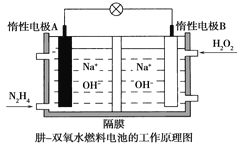
(4)��-˫��ˮȼ�ϵ��������ϸߵ������ܶȶ����ܹ�ע���乤��ԭ����ͼ��ʾ������������ӦʽΪ_____________________����ع��������У�A������Һ��pH___________________���������С�����䡱��
���𰸡� 2 N2H4+4Cu(OH)2=2Cu2O+N2+6H2O N2H4(l)+2H2O2(l)=N2(g)+4H2O(g) ��H=-1284.4kJ/mol H2O2+2e-=2OH- ����
��������(1)1mol���к���4molN-H���Թ��ۼ�����0.5mol���к���2mol���Թ��ۼ����ʴ�Ϊ��2��
(2)����Cu(OH)2����(N2H4)��Ӧ���Ʊ�����Cu2O��ͬʱ�ų�N2���������غ㶨�ɣ���Ӧǰ��Ԫ������䣬��˷�Ӧ������ˮ��Cu2O�Ļ�ѧ������Ϊ2����Ӧ�Ļ�ѧ����ʽΪ��N2H4+4Cu(OH)2=2Cu2O+N2��+6H2O��
(3)��Ӧ����ʽΪ��N2H4+2H2O2�TN2+4H2O��1.6gҺ̬�µ����ʵ���Ϊ![]() = 0.05mol���ų�64.22kJ ����������1molҺ̬�·ų�������Ϊ
= 0.05mol���ų�64.22kJ ����������1molҺ̬�·ų�������Ϊ![]() =1284.4kJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4(l)+2H2O2(l)�TN2(g)+4H2O(g)��H=-1284.4kJ/mol���ʴ�Ϊ��N2H4(l)+2H2O2(l)=N2(g)+4H2O(g)��H=-1284.4kJ/mol��
=1284.4kJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4(l)+2H2O2(l)�TN2(g)+4H2O(g)��H=-1284.4kJ/mol���ʴ�Ϊ��N2H4(l)+2H2O2(l)=N2(g)+4H2O(g)��H=-1284.4kJ/mol��
(4)��-˫��ˮȼ�ϵ����ͨ���µ��Ǹ�����ͨ��˫��ˮ��������������������ԭ��Ӧ������������ӦʽΪH2O2+2e-=2OH-����ع��������У�A��Ϊ�������缫��ӦʽΪN2H4-4e-+4OH-=N2+4H2O����������Һ��pH��С���ʴ�Ϊ��H2O2+2e-=2OH-�����١�

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д� ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д�