��Ŀ����
��ͼ��,AΪ�����г��������嵥�ʡ�B��C��E�ǽ�������,DΪ�ǽ������ʡ���֪:��I��һ�ֳ�������������,Eԭ�Ӻ�����12�����Ӣ�GΪ��ɫ����,��ӦC+G B+H�ܷų���������,�÷�Ӧ��Ӧ��������ĺ���;�ش���������:
B+H�ܷų���������,�÷�Ӧ��Ӧ��������ĺ���;�ش���������:
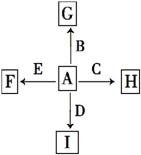
(1)�ֱ�д��F��G��H��I�Ļ�ѧʽ:
F________��G________��H________��I_______
(2)��д���л�ѧ����ʽ:
C+G B+H___________________________________________________;
B+H___________________________________________________;
2 E+I
E+I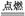 2F+D__________________________________________________��
2F+D__________________________________________________��
(3)C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ___________________________,��Ӧ����Һ�����������I��Ӧ�Ļ�ѧ����ʽΪ____________________________��
(4)1.6 g G��������,�õ�����Һ��ͭ����ȫ��Ӧ,�������������ͭ�۵�����Ϊ:________��
.���������������֪�ɵ�:IΪCO2,EΪMg,GΪFe2O3,HΪAl2O3,CΪAl,BΪFe,����AΪO2,FΪMgO,DΪC��
m(Fe2O3)=1.6 g/160 g��mol-1=0.01 mol
Fe2O3+��6HCl====��2FeCl3����+����3H2O
0.01 mol����������0.02 mol
2FeCl3��+��Cu====2FeCl2+CuCl2
0.02 mol ��0.01 mol
m(Cu)=0.01 mol��64 g��mol-1=0.64 g��
��:(1)MgO��Fe2O3��Al2O3��CO2
(2)2Al+Fe2O3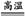 2Fe+Al2O3
2Fe+Al2O3
2Mg+CO2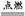 2MgO+C
2MgO+C
(3)2Al+2NaOH+6H2O====2Na[Al(OH)4]+3H2��
Na[Al(OH)4]+CO2====Al(OH)3��+NaHCO3
(4)0.64 g

 ��������������������ϵ�д�
��������������������ϵ�д�
 2C(g)��2D(g)���ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ�ķ�Ӧ���������� (����)
2C(g)��2D(g)���ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ�ķ�Ӧ���������� (����)
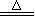 CuSO4��SO2����2H2O�����ڸ÷�Ӧ������˵���в���ȷ����
CuSO4��SO2����2H2O�����ڸ÷�Ӧ������˵���в���ȷ����