��Ŀ����
(10��)������ҵ�ǹ��ҹ�ҵ�Ļ�������ش������ʴ�����������е��й����⡣
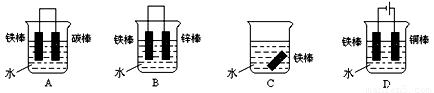
��1�����ڳ�ʪ�Ŀ��������ױ���ʴΪ���⣨Fe2O3•x H2O����д���������绯ѧ��ʴʱ�����ĵ缫��Ӧ��___________________________________ ��
��2�����и���װ������������ʴ���ѵ���˳����_______________������ĸ����
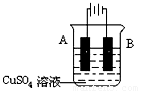
��3����ʵ�������У����������ı����ͭ��ֹ������ʴ��װ����ͼ����ش�
�� A�缫�ĵ缫��Ӧʽ�� __
�� �����ǰ����ͭ���缫��������ͬ�������ɺ�, �����ʱ
��·��ͨ���ĵ���Ϊ0.2mol��������ȡ��ϴ������ɡ���������A��B�����������____g��
�� �Ʋ������ͭ���ȶ�п�������ױ���ʴ�����Ҫ˵��ԭ��
��
(10��)��1��Fe ��2e�� �� Fe2�� ��2��D BCA
��3����Cu��2e- =Cu2+ �� 12.8g
������ͭ���ã��Ʋ��ƻ����ڳ�ʪ�������γ�ԭ��أ���Ϊ�������������ĸ�ʴ��
����:

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���07�꽭�վ���(10��)������ҵ�ǹ��ҹ�ҵ�Ļ�����2006���ҹ��ֲָ���ͻ��4�ڶ֣���������λ��ij��ѧ���ʵ���С�����ü��ڶԵ��ظ����������˵��У��Դӿ�ʯ��ʼ�����������Ĺ�����������ȫ��ĸ�����ʶ�����������ʵ���С�����Ȥ��������м��㣺
(1)��6��62 g����ʯ��ƷͶ��������������(��ַ�Ӧ)�����ˣ�Ȼ������Һ�мӹ�����NaOH ��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����յ�4.80g Fe2O3�����Ը�����ʯΪԭ����������������������Ԫ����ʧ4��������ÿ����1.00t����(����96��)��������Ҫ��������ʯ���ٶ�? (������λС��)
(2)ȡij������ĩ28.12g(����ֻ��Fe��C)�����������г�ַ�Ӧ���õ�CO2����224mL(��״��)��
�� ����˸�������������̼�����ʵ���֮�ȡ�
����ȡ���ݲ�ͬ�����ĸ�����ĩ �ֱ�ӵ�100mL��ͨŨ�ȵ�H2SO4��Һ�У���ַ� Ӧ�� ��õ�ʵ���������±���ʾ��
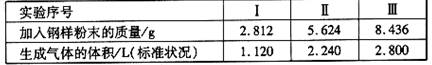 |
����������Һ�����ʵ���Ũ�ȡ�
������ʵ����м�������mg������ĩ�����㷴Ӧ������ʣ��Ĺ�������Ϊ����?(�ú�m�Ĵ���ʽ��ʾ)
����Hg��������Cr����Ӱ�컷����Ⱦ������ЧӦ�����ཡ�����ؽ���Ԫ�ء�
I�����㷺Ӧ���ڸ�����ҵ���Ŵ���¼����ȷ��档
��1����ҵ�����á����ڸ��������»�ԭ���̣�Cr2O3�����Ʊ������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
II�������Ĺ�ҵ��ˮ�ᵼ�������ж���������+6�۸��ķ�ˮʱ�ɵõ������壨����ɿ�д��[Fe2+Fe3+(2-x) Cr3+x]O4����

��2���ӹ���FeSO4��Ŀ���� ��
��3����ƽ����ٵķ�Ӧ�����ӷ���ʽ Fe2++ Cr2O72��+ _____== Fe3++ Cr3++ H2O
�����������壨 [Fe2+Fe3+(2-x) Cr3+x]O4����X= _________________��
��4���±���ʵ�������Ķ�ij�����������������Ʒ��ÿ���������ȡ������6����Ʒ���������л��ʺ������IJⶨ��������±���
|
��Ʒ��� |
ȡ�����(m) |
���(��10��2g) |
�ܸ�(��10��6g) |
|
��ƷA-1 |
0.00 ~ 0.30 |
2.81 |
114 |
|
��ƷA-2 |
0.30 ~ 0.60 |
1.72 |
111 |
|
��ƷA-3 |
1.20 ~ 1.80 |
1.00 |
88 |
|
��ƷB-1 |
0.00 ~ 0.30 |
2.60 |
116 |
|
��ƷB-2 |
0.30 ~ 0.60 |
2.48 |
112 |
|
��ƷB-3 |
1.20 ~ 1.80 |
1.83 |
106 |
�ӱ�������Եó��Ľ����� ����һ�㼴�ɣ���
��5����ҵ���Ը���أ�K2CrO4��Ϊԭ�ϣ��绯ѧ���Ʊ��ظ���أ�װ����ͼ����ӦΪ��4K2CrO4+4H2O 2K2Cr2O7+4KOH+2H2��+O2����֪K2CrO4�������Һ�д���ƽ�⣺2CrO42��(��ɫ)+2H��
2K2Cr2O7+4KOH+2H2��+O2����֪K2CrO4�������Һ�д���ƽ�⣺2CrO42��(��ɫ)+2H�� Cr2O72��(��ɫ)+H2Oͨ�����������Һ�� ��Ϊ
��ԭ����
��
Cr2O72��(��ɫ)+H2Oͨ�����������Һ�� ��Ϊ
��ԭ����
��