��Ŀ����
ʹһ����������������Ļ�����壬����ͨ����֧���ȵ�Ӳ�ʲ����ܣ���1֧��������װ��ͭм����2֧��������װ������ͭ����1֧�����������ڷ������·�Ӧ��2PH3+Cu Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g��
Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g����1������ԭ������������������������ȣ�
��2���ڱ�״���£�ԭ���������ܶ��Ƕ��٣�
���𰸡���������1����1֧�Թ��з�����Ӧ2PH3+Cu Cu3P2��s��+3H2���ɷ���ʽ��֪�������ص���������Ԫ�����������ݹ������ؼ���PH3�����ʵ������������������ʵ�����
Cu3P2��s��+3H2���ɷ���ʽ��֪�������ص���������Ԫ�����������ݹ������ؼ���PH3�����ʵ������������������ʵ�����
��2֧�Թ��з�����ӦH2+CuO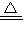 Cu+H2O���ɷ���ʽ��֪������ٵ�����Ϊ����ͭʧȥ��Ԫ��������ʧȥ�����������������ˮ����2֧�Թ�����������ԭ��������������͵�1֧�Թ������ɵ������������������ټ����2֧�Թ����������ʵ�������ȥ1֧�Թ������ɵ�������Ϊԭ���������������
Cu+H2O���ɷ���ʽ��֪������ٵ�����Ϊ����ͭʧȥ��Ԫ��������ʧȥ�����������������ˮ����2֧�Թ�����������ԭ��������������͵�1֧�Թ������ɵ������������������ټ����2֧�Թ����������ʵ�������ȥ1֧�Թ������ɵ�������Ϊԭ���������������
��2�����ݣ�1�������������ƽ��Ħ�������������æ�=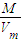 �����ܶȣ�
�����ܶȣ�
����⣨1��2PH3+3Cu��Cu3P2��s��+3H2 ���ӵ�����
2mol 3mol 62g
n��PH3�� n��H2�� 4.96g
����n��PH3��=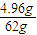 ×2mol=0.16mol��
×2mol=0.16mol��
n��H2��=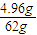 ×3mol=0.24mol��
×3mol=0.24mol��
H2+CuO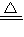 Cu+H2O ���ٵ�����
Cu+H2O ���ٵ�����
1mol 16g
n�䣨H2�� 5.76g
���� n�䣨H2��=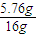 ×1mol=0.36mol��
×1mol=0.36mol��
����ԭ�����������������ʵ���Ϊ0.36mol-0.24mol=0.12mol��
��ԭ�������������������������Ϊ0.16mol��0.12mol=4��3��
��ԭ������������������������� 4��3
��2��ԭ�������ƽ��Ħ������=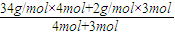 =
=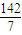 mol/L=20.3g/mol
mol/L=20.3g/mol
�ڱ�״���£�ԭ���������ܶȦ�=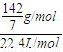 =0.91g/L
=0.91g/L
���ڱ�״���£�ԭ���������ܶ�0.91g/L��
�����������йػ����ļ��㣬���ݷ���ʽ���ò��������㣬�Ѷ��еȣ�ע���2֧�Թ�����������ԭ��������������͵�1֧�Թ������ɵ�������
 Cu3P2��s��+3H2���ɷ���ʽ��֪�������ص���������Ԫ�����������ݹ������ؼ���PH3�����ʵ������������������ʵ�����
Cu3P2��s��+3H2���ɷ���ʽ��֪�������ص���������Ԫ�����������ݹ������ؼ���PH3�����ʵ������������������ʵ�������2֧�Թ��з�����ӦH2+CuO
 Cu+H2O���ɷ���ʽ��֪������ٵ�����Ϊ����ͭʧȥ��Ԫ��������ʧȥ�����������������ˮ����2֧�Թ�����������ԭ��������������͵�1֧�Թ������ɵ������������������ټ����2֧�Թ����������ʵ�������ȥ1֧�Թ������ɵ�������Ϊԭ���������������
Cu+H2O���ɷ���ʽ��֪������ٵ�����Ϊ����ͭʧȥ��Ԫ��������ʧȥ�����������������ˮ����2֧�Թ�����������ԭ��������������͵�1֧�Թ������ɵ������������������ټ����2֧�Թ����������ʵ�������ȥ1֧�Թ������ɵ�������Ϊԭ�����������������2�����ݣ�1�������������ƽ��Ħ�������������æ�=
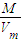 �����ܶȣ�
�����ܶȣ�����⣨1��2PH3+3Cu��Cu3P2��s��+3H2 ���ӵ�����
2mol 3mol 62g
n��PH3�� n��H2�� 4.96g
����n��PH3��=
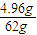 ×2mol=0.16mol��
×2mol=0.16mol��n��H2��=
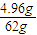 ×3mol=0.24mol��
×3mol=0.24mol��H2+CuO
 Cu+H2O ���ٵ�����
Cu+H2O ���ٵ�����1mol 16g
n�䣨H2�� 5.76g
���� n�䣨H2��=
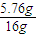 ×1mol=0.36mol��
×1mol=0.36mol������ԭ�����������������ʵ���Ϊ0.36mol-0.24mol=0.12mol��
��ԭ�������������������������Ϊ0.16mol��0.12mol=4��3��
��ԭ������������������������� 4��3
��2��ԭ�������ƽ��Ħ������=
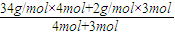 =
=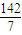 mol/L=20.3g/mol
mol/L=20.3g/mol�ڱ�״���£�ԭ���������ܶȦ�=
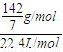 =0.91g/L
=0.91g/L���ڱ�״���£�ԭ���������ܶ�0.91g/L��
�����������йػ����ļ��㣬���ݷ���ʽ���ò��������㣬�Ѷ��еȣ�ע���2֧�Թ�����������ԭ��������������͵�1֧�Թ������ɵ�������

��ϰ��ϵ�д�
�����Ŀ
 Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g��
Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g��