��Ŀ����
���г����µ��ķ���Һ����0��01 mol��L CH3COOH��Һ����0��0l mol��L HC1��Һ����pH=12�İ�ˮ����pH=12��NaOH��Һ������˵����ȷ����
| A������ˮ�ĵ���̶���С������ˮ�ĵ���̶���� |
| B�����ڡ��ۻ�ϣ���pH=7����������Һ���������>�� |
| C�����١��ܻ�ϣ���c(CH3COO-)>c(H+)������Һһ���ʼ��� |
| D�����ķ���Һ�ֱ�ϡ��100������Һ��pH:��>�ܣ���<�� |
B
�������������A���٢ڢۢ���ȣ����е�������Ũ����С����ˮ�ĵ���Ӱ�����������Ԣ���ˮ�ĵ���̶������B��pH=12�İ�ˮ��һˮ�ϰ���Ũ�ȴ���0.01mol/L�����Ե�����Ģڢۻ�ϣ���Һ���Լ��ԣ�����Ҫʹ��Һ�����ԣ���ӦС��������������>��,��ȷ��C���٢ܵ�����Ũ����ȣ�����ϣ�������Һ��Ϊ����ʹ����Ƶ���Һʱ����c(CH3COO-)>c(H+)����������ʱ��ҺΪ���ԣ�����D�����ڢۢܣ�pH��ͬ��ϡ����ͬ�ı���������һˮ�ϰ��������ٽ�����룬����ˮ�ļ���ǿ���������ƣ����ڢ٢ڣ����ߵ����ʵ���Ũ����ͬ�����е�������Ũ��ԶԶ���ڢ٣�����ϡ����ͬ�ı������ܻ�ٽ����еĴ�����ӵĵ��룬��������Ũ���Ի�С�ڢڣ����Ԣ٢���ȣ��ڵ�pHС�ڢ٣����Ը�ǿ�����Դ���ѡB��
���㣺����ǿ�����������Һ�е�����Ũ�������ʵ���Ũ�ȵĹ�ϵ��ϡ�ͺ����ҺpH�仯����Һ����Ե��ж�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�������Һ�У���Ũ�ȹ�ϵ��ȷ����( )
| A������ NH4+��Cl-��H+��OH-����Һ�У�������Ũ�ȿ�����c(Cl-)> c(NH4+)>c(OH-)>c(H+) |
| B��������pH=6�Ĵ���������ƵĻ����Һ��c(Na+)>c(CH3COO-) |
| C��0��1 mol��L-1��Na2S��Һ�У�c(OH-)=c(H+)+c(HS-)+2c(H2S) |
| D��������pH=3��һԪ���pH=11��һԪ��������Ϻ����Һ��һ����c(OH-)=c(H+) |
���ʵ���Ũ����ͬ��������Һ�У����ϰ�pH��С����˳�����е��ǣ� ��
| A��Na2CO3��NaHCO3��NaCl��NH4Cl |
| B��NH4Cl��NaCl ��Na2CO3��NaHCO3 |
| C��(NH4)2SO4��NH4Cl��NaNO3��Na2S |
| D��NH4Cl ��(NH4)2SO4��Na2S ��NaNO3 |
��һ���¶��£�һ������ˮ�У�ʯ��������Һ��������ƽ�⣺Ca(OH)2(s�� Ca(OH)2(aq��
Ca(OH)2(aq�� Ca2+(aq)+2OH-(aq)�����������Һ�м���������ʯ��ʱ������˵����ȷ����( )
Ca2+(aq)+2OH-(aq)�����������Һ�м���������ʯ��ʱ������˵����ȷ����( )
| A��n(Ca2+)���� | B��c(Ca2+)���� | C��n(OH-)���� | D��c(OH-)��С |
��ˮΪ�ܼ������к͵ζ���ԭ���ǣ�H3O����OH��=2H2O����֪Һ̬SO2�ʹ�ˮ�ĵ������������ΪҺ̬SO2Ҳ�ᷢ������⣺SO2(l)��SO2(l)  SO32-��SO2��������Һ̬SO2Ϊ�ܼ�����SOCl2�ζ�Cs2SO3�������������������( )
SO32-��SO2��������Һ̬SO2Ϊ�ܼ�����SOCl2�ζ�Cs2SO3�������������������( )
| A���õζ���Ӧ���Ա�ʾΪ��SO32-��SO2��=2SO2 |
| B����һ���¶��£�Һ̬SO2��c(SO32-)��c(SO2��)�ij˻���һ������ |
| C�������Ĵ��ڣ�˵��SO2�����ӻ����� |
| D����ͨ�����������仯���ж��Ƿ�ζ��յ� |
�����йص������Һ��˵����ȷ����
| A��������ˮ�еμ�ŨH2SO4��Kw���� |
B��CH3COOH��Һ��ˮϡ�ͺ���Һ��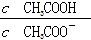 ��ֵ��С ��ֵ��С |
C��Na2CO3��Һ�м�������Ca(OH)2���壬 ˮ��̶ȼ�С����Һ��pH��С ˮ��̶ȼ�С����Һ��pH��С |
| D��NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
����10 mL 0.1 mol��L��1��NH3��H2O��Һ������˵����ȷ����
| A��25 ��ʱ������Һ��pH��11����Kb(NH3��H2O)��1��10��6 mol��L��1 |
B������Һ�м�������CH3COONa���壬��Һ�е�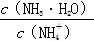 ֵ��С ֵ��С |
C������Һ�м���10 mL 0.1 mol��L��1 HCl��������Һ������Ũ�ȴ�С˳��Ϊc(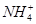 )>c(Cl��)>c(H��)>c(OH��) )>c(Cl��)>c(H��)>c(OH��) |
D������Һ�м���5 mL 0.1 mol��L��1 HCl��������Һ�����ӵ�Ũ��һ�����ϣ�c(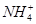 )��c(H��)��c(Cl��)��c(OH��) )��c(H��)��c(Cl��)��c(OH��) |
���й�����Һ������ԣ�˵����ȷ����(����)
| A��pH��7����Һ������ |
| B��������Һ��һ����c(H��)��1.0��10��7 mol��L��1 |
| C��c(H��)��c(OH��)����Һ������ |
| D����100��Cʱ����ˮ��pH��7����������� |
��֪�������£�Ksp(AgCl)��1.8��10��10��Ksp(AgI)��8.5��10��17�����������У���ȷ����(����)
| A�������£�AgCl�ڱ���NaCl��Һ�е�Ksp���ڴ�ˮ�е�KspС |
| B����AgCl������Һ�м���KI��Һ�������ɰ�ɫת��Ϊ��ɫ |
| C����0.001 mol��L��1��AgNO3��Һ����KCl��KI�Ļ����Һ�У�һ���Ȳ���AgI���� |
| D����AgCl�ı�����Һ�м���NaCl���壬��AgCl��������Һ��c(Ag��)��c(Cl��) |