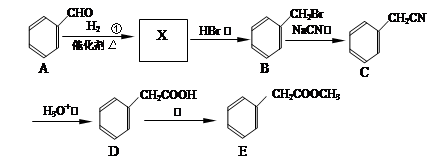
��Ŀ����
���ƽ�����ھ��������ƣ���ṹΪ��
(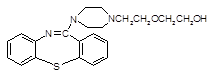 )2��
)2�� �����ĺϳ�·�����£�
�����ĺϳ�·�����£�
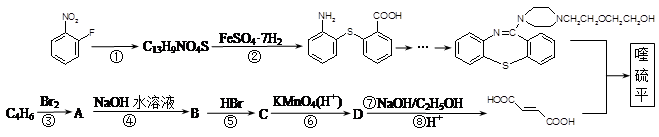
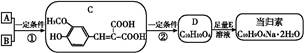
��֪������Ӧ��Ϊȡ����Ӧ������A��ϵͳ����Ϊ1,4������D2�D��ϩ��
��ش��������⣺
��1����Ӧ�ٵIJ���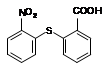 �г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��
�г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��
��2����Ӧ�۵������� ��Ӧ����Ӧ�ݵ�Ŀ���� ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ ��
��4������B��ͬ���칹���ж��֣����мȺ����ǻ����ֺ���ȩ����ͬ���칹���� �֡�
��5����֪����SH�������룭OH���ơ�
����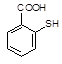 һ���������γɾۺ���Ľṹ��ʽΪ ��
һ���������γɾۺ���Ľṹ��ʽΪ ��
(
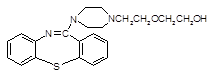 )2��
)2�� �����ĺϳ�·�����£�
�����ĺϳ�·�����£�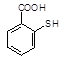
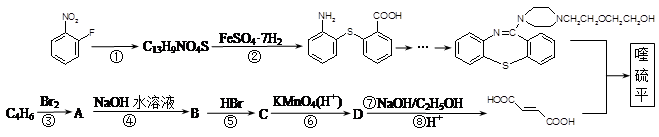
��֪������Ӧ��Ϊȡ����Ӧ������A��ϵͳ����Ϊ1,4������D2�D��ϩ��
��ش��������⣺
��1����Ӧ�ٵIJ���
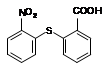 �г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��
�г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ����2����Ӧ�۵������� ��Ӧ����Ӧ�ݵ�Ŀ���� ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ ��
��4������B��ͬ���칹���ж��֣����мȺ����ǻ����ֺ���ȩ����ͬ���칹���� �֡�
��5����֪����SH�������룭OH���ơ�
����
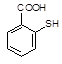 һ���������γɾۺ���Ľṹ��ʽΪ ��
һ���������γɾۺ���Ľṹ��ʽΪ ����1���������Ȼ�
��2���ӳɣ�����̼̼˫������ֹ������KMnO4����
��3��BrCH2CH��CHCH2Br��2NaOH HOCH2CH��CHCH2OH��2NaBr
HOCH2CH��CHCH2OH��2NaBr
��4��5
��5��
��2���ӳɣ�����̼̼˫������ֹ������KMnO4����
��3��BrCH2CH��CHCH2Br��2NaOH
 HOCH2CH��CHCH2OH��2NaBr
HOCH2CH��CHCH2OH��2NaBr ��4��5
��5��

�����������1����
 ��֪C13H9NO4S�����ʵĽṹ��ʽΪ
��֪C13H9NO4S�����ʵĽṹ��ʽΪ ��
�� �л�����-NO2��-COOH�����ţ��ֱ�Ϊ�������Ȼ�����2��C4H6����ӦΪCH2=CH-CH=CH2�����巢��1��4�ӳɣ�����CH2Br-CH=CH-CH2Br����Ӧ�ݵ�Ŀ���Ƿ�ֹC=C�����Ը���������������������ŵ����ã��ʴ�Ϊ���ӳɷ�Ӧ������̼̼˫������ֹ������KMnO4��������3��AΪCH2Br-CH=CH-CH2Br���ڼ��������·���ȡ����Ӧ����HOCH2CH=CHCH2OH����Ӧ�Ļ�ѧ����ʽΪBrCH2CH��CHCH2Br��2NaOH
�л�����-NO2��-COOH�����ţ��ֱ�Ϊ�������Ȼ�����2��C4H6����ӦΪCH2=CH-CH=CH2�����巢��1��4�ӳɣ�����CH2Br-CH=CH-CH2Br����Ӧ�ݵ�Ŀ���Ƿ�ֹC=C�����Ը���������������������ŵ����ã��ʴ�Ϊ���ӳɷ�Ӧ������̼̼˫������ֹ������KMnO4��������3��AΪCH2Br-CH=CH-CH2Br���ڼ��������·���ȡ����Ӧ����HOCH2CH=CHCH2OH����Ӧ�Ļ�ѧ����ʽΪBrCH2CH��CHCH2Br��2NaOH HOCH2CH��CHCH2OH��2NaBr;��4��BΪOH-CH2-CH=CH-CH2-OH���Ⱥ����ǻ����ֺ���ȩ����ͬ���칹���У�CH2��OH��CH2CH2CHO��CH3CH��OH��CH2CHO��CH3CH2CH��OH��CHO��HOCH2CH��CH3��CHO��CH2C��OH����CH3��CHO�����Թ���5�֣���5������
HOCH2CH��CHCH2OH��2NaBr;��4��BΪOH-CH2-CH=CH-CH2-OH���Ⱥ����ǻ����ֺ���ȩ����ͬ���칹���У�CH2��OH��CH2CH2CHO��CH3CH��OH��CH2CHO��CH3CH2CH��OH��CHO��HOCH2CH��CH3��CHO��CH2C��OH����CH3��CHO�����Թ���5�֣���5������ ��һ�������·������۷�Ӧ��������������Ľṹ��ʽΪ��
��һ�������·������۷�Ӧ��������������Ľṹ��ʽΪ�� ��
��
��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ

 CH2�Ƶ��л���A�Ļ�ѧ����ʽΪ������������������,��Ӧ����������������
CH2�Ƶ��л���A�Ļ�ѧ����ʽΪ������������������,��Ӧ���������������� 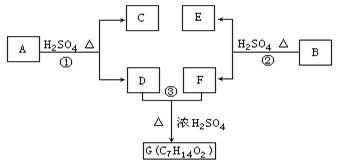
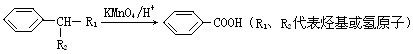
 RCH
RCH C��COOH��2+H2O��RCH
C��COOH��2+H2O��RCH RCH
RCH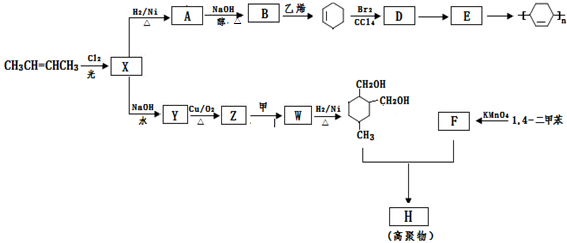

 ��Z��W ��
��Z��W ��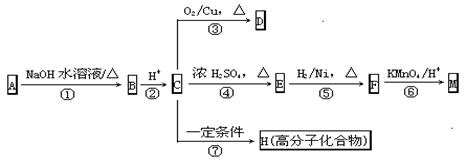
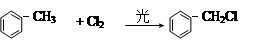 �� R��CH2��CH = CH2 + Cl2
�� R��CH2��CH = CH2 + Cl2 R��CHCl��CH = CH2 + HCl
R��CHCl��CH = CH2 + HCl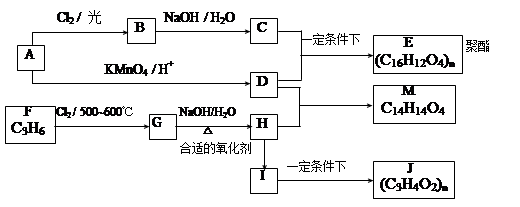
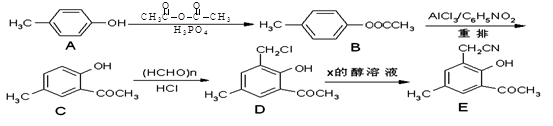
 R-COOH��E������������ˮ���IJ�����һ�������¿�����F��C11H10O3����д��F�Ľṹ��ʽ�� ��
R-COOH��E������������ˮ���IJ�����һ�������¿�����F��C11H10O3����д��F�Ľṹ��ʽ�� ��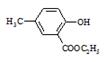 �ĺϳ�·������ͼ��
�ĺϳ�·������ͼ�� CH3CH2OH
CH3CH2OH 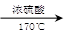 H2C��CH2 BrH2C��CH2Br
H2C��CH2 BrH2C��CH2Br