��Ŀ����
��9�֣�ij��ȤС��ͬѧ������ȩ��������Ӧ��ʵ������������£�
��1������A�м�NaOH��Һ��������е�Ŀ���ǣ� �� ���������� ������ ��
���������� ������ ��
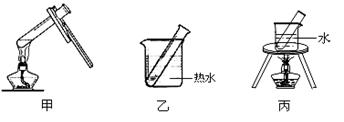
��2������DӦѡ��ļ��ȷ����� ��������װ�ñ�ţ�
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ������������
��4������ȤС���ͬѧ������ȩ����������Ӧ�����ʵ������������̽����ʵ�����±��� ��
��
��ʵ��1��ʵ��2��̽������ ��
�ڵ�������Һ����Ϊ1 mL����ȩ����Ϊ3�Σ��¶�Ϊ55�棬��Ӧ���ҺpHΪ11ʱ������������ʱ��Ϊ min�����Χ��
������Ϊ̽����ȩ����������Ӧ��������������˲ⶨ�������ֵ�ʱ���⣬����Ҫ�Ƚϲ�ͬ�������γɵ������� ��
| A�����Թ�����ע������NaOH��Һ����Ȼ�������С���NaOH��Һ��ȥ����������ˮϴ���Թܱ��á� | ||
| B����ϴ�����Թ�������������Һ�� | C�����Թܱڼ�����ȩϡ��Һ�� | D�����ȡ���ش��������⣺ |
 ���������� ������ ��
���������� ������ ����2������DӦѡ��ļ��ȷ����� ��������װ�ñ�ţ�

��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ������������
��4������ȤС���ͬѧ������ȩ����������Ӧ�����ʵ������������̽����ʵ�����±���
 ��
��
| ʵ����� ʵ����� | ������Һ����/mL | ��ȩ����/�� | �¶�/�� | ��Ӧ���Һ��pH | ����������ʱ��/min |
| 1 | 1 | 3 | 65 | 11 | 5 |
| 2 | 1 | 3 | 45 | 11 | 6.5 |
| 3 | 1 | 5 | 65 | 11 | 4 |
�ڵ�������Һ����Ϊ1 mL����ȩ����Ϊ3�Σ��¶�Ϊ55�棬��Ӧ���ҺpHΪ11ʱ������������ʱ��Ϊ min�����Χ��
������Ϊ̽����ȩ����������Ӧ��������������˲ⶨ�������ֵ�ʱ���⣬����Ҫ�Ƚϲ�ͬ�������γɵ������� ��
��9�֣�
��1��ȥ���Թ��ڱڵ����ۣ���֤�Թܽྻ(2��)
��2����(2��)
��3��CH3CHO + 2Ag(NH3)2OH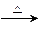 CH3COONH4 + 2Ag��+ 3NH3��+ H2O (2��)
CH3COONH4 + 2Ag��+ 3NH3��+ H2O (2��)
��4�����¶ȶԷ�Ӧ���ʵ�Ӱ��(1��) �� 5��6.5 (1��) �۹����̶�(1��)
��1��ȥ���Թ��ڱڵ����ۣ���֤�Թܽྻ(2��)
��2����(2��)
��3��CH3CHO + 2Ag(NH3)2OH
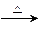 CH3COONH4 + 2Ag��+ 3NH3��+ H2O (2��)
CH3COONH4 + 2Ag��+ 3NH3��+ H2O (2��)��4�����¶ȶԷ�Ӧ���ʵ�Ӱ��(1��) �� 5��6.5 (1��) �۹����̶�(1��)
��

��ϰ��ϵ�д�
�����Ŀ
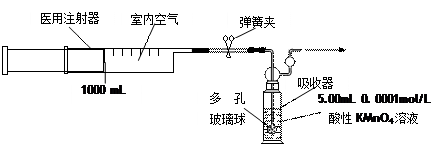
 �л���X��ȫȼ�����ɵ����ʵ�����CO2��H2O��ͬʱ���ı�״����O2112L��
�л���X��ȫȼ�����ɵ����ʵ�����CO2��H2O��ͬʱ���ı�״����O2112L��
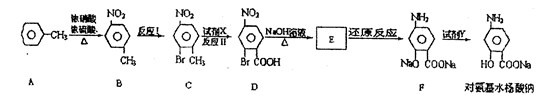
 ��
��