��Ŀ����
��26�֣����й���������������һ�ݿƼ����ؿ����о������ʾ���ҹ���������ռ���������40%���ϡ��о�������������ҹ�ũҵÿ�������������ɵ���ʧ��15��Ԫ��Ϊ����Ч�������꣬Ŀǰ����Ժ����������������Ͷ���������Ⱦ���������ַ������ȷ��档
(1)��Ӣ�����е�һ���о�������������̴ѿ���Ч�ؽ��͵���SO2Ũ�ȡ���20���͵�60��70�����10��䣬�ɷ��糧�ŷŵ�SO2������35%�������ڽ�����̴ѵĽ��������Ũ�Ƚ�����30%֮�ࡣ�����ȫ�������ĽǶȣ��������������Ƿ��ȡ?���������ɡ�
(2)�ô�ͳ��ú��ʯ����ȼ�ϣ�����Ҫȱ����ʲô?�봫ͳ��ú��ʯ��ȼ����ȣ��������ʿ�����Ϊ����Դ?��Ҫ�ŵ���ʲô��ȱ������ʲô?
(3)�о���������õġ����������������������в����ܵĴֵ��ߴ�����Ƥ�����̶��ڲ������У�����һ���õ���˿�������������Ƴɡ���������ʵ��ʱ�Ƚ�ֱͨ����Դ��ʹ����˿���ȣ�Ȼ���ȵĵ���˿����װ��SO2�Ϳ����ļ���ƿ�У�ƿ����������a�����������м����������ữ���BaCl2��Һ���ֳ���b������ش�
������a______________________________��
b_______________________________________��
�ڴ�����ʵ��ɵó��������Ļ�ѧԭ��
___________________________________________��
(4)Ŀǰһ���еȳ���ÿ����úԼ300��֡��京������1%���㣬��ÿ���ŷ�SO2���ٶ�?����SO2��60%ת��Ϊ���ᣬ�൱�����ɶ��ٶ�98%������?
(1)��Ӣ�����е�һ���о�������������̴ѿ���Ч�ؽ��͵���SO2Ũ�ȡ���20���͵�60��70�����10��䣬�ɷ��糧�ŷŵ�SO2������35%�������ڽ�����̴ѵĽ��������Ũ�Ƚ�����30%֮�ࡣ�����ȫ�������ĽǶȣ��������������Ƿ��ȡ?���������ɡ�
(2)�ô�ͳ��ú��ʯ����ȼ�ϣ�����Ҫȱ����ʲô?�봫ͳ��ú��ʯ��ȼ����ȣ��������ʿ�����Ϊ����Դ?��Ҫ�ŵ���ʲô��ȱ������ʲô?
(3)�о���������õġ����������������������в����ܵĴֵ��ߴ�����Ƥ�����̶��ڲ������У�����һ���õ���˿�������������Ƴɡ���������ʵ��ʱ�Ƚ�ֱͨ����Դ��ʹ����˿���ȣ�Ȼ���ȵĵ���˿����װ��SO2�Ϳ����ļ���ƿ�У�ƿ����������a�����������м����������ữ���BaCl2��Һ���ֳ���b������ش�
������a______________________________��
b_______________________________________��
�ڴ�����ʵ��ɵó��������Ļ�ѧԭ��
___________________________________________��
(4)Ŀǰһ���еȳ���ÿ����úԼ300��֡��京������1%���㣬��ÿ���ŷ�SO2���ٶ�?����SO2��60%ת��Ϊ���ᣬ�൱�����ɶ��ٶ�98%������?
(1)����ȡ����ΪSO2���ŷ�������û�м��٣���һ���γɵ������Ի���ɶ�ȫ���Σ����
(2)ú��ʯ���Dz��������Ļ�ʯȼ�ϣ�����Դ�����ģ����ȼ�պ������SO2��NO2��������Ⱦ�����������γ����꣬ȼ�պ������CO2�ֻ��������ЧӦ������ȼ�ϣ���һ��H2������ˮ��Ϊԭ������ȡ�����ȼ��ʱ���ȶ࣬�ų�������ԼΪͬ�������͵���������ȼ�ϵ�����ŵ���ȼ�ղ���Ϊˮ��������Ⱦ����������ѭ��ʹ�á�ȱ���ǣ������ڴ��������䡣
(3)��Ũ�������(H2SO4) ��ɫ���� �ڿ����е�SO2������ú������SO2�Ĺ�������þͻ��γ�����
(4)��һ���ŷŵ�SO2����Ϊx������98�����������Ϊy��
S �� SO2 �� H2SO4
32 t 64 t 98 t
3��106��1% t x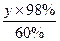
x="60" 000 t
y="56" 300 t
(2)ú��ʯ���Dz��������Ļ�ʯȼ�ϣ�����Դ�����ģ����ȼ�պ������SO2��NO2��������Ⱦ�����������γ����꣬ȼ�պ������CO2�ֻ��������ЧӦ������ȼ�ϣ���һ��H2������ˮ��Ϊԭ������ȡ�����ȼ��ʱ���ȶ࣬�ų�������ԼΪͬ�������͵���������ȼ�ϵ�����ŵ���ȼ�ղ���Ϊˮ��������Ⱦ����������ѭ��ʹ�á�ȱ���ǣ������ڴ��������䡣
(3)��Ũ�������(H2SO4) ��ɫ���� �ڿ����е�SO2������ú������SO2�Ĺ�������þͻ��γ�����
(4)��һ���ŷŵ�SO2����Ϊx������98�����������Ϊy��
S �� SO2 �� H2SO4
32 t 64 t 98 t
3��106��1% t x
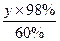
x="60" 000 t
y="56" 300 t
�����漰��Դ������������ȵ����⣬����Ҫ�ӻ�ѧ�ӽ�ȥ��ע��Щ����̬�����������ǵĽ���������ص����⡣������Ⱦ�Ķ����ֱ��Ӱ������������ͷ�չ��������Ӱ��ȫ�����Ⱦ���⡣?
��������IJ�����Ҫ�ž���ȾԴ�������ǽ�����̴�ת����Ⱦ�����ž��������Ⱦ;��һ����������һ�Ƕ�ú��ʯ�͵ȴ�ͳ��Դ����Ԥ������ʹ����ȼ�չ����в�����SO2�����ǽ��л������ã����ǿ�������Դ����Ŀǰ���������ԴΪ����Դ����Ϊ��ԭ����Դ�ḻ��ȼ�յIJ�������ˮ���Ȳ���Ⱦ�������ֿ���ѭ�����á�
��������IJ�����Ҫ�ž���ȾԴ�������ǽ�����̴�ת����Ⱦ�����ž��������Ⱦ;��һ����������һ�Ƕ�ú��ʯ�͵ȴ�ͳ��Դ����Ԥ������ʹ����ȼ�չ����в�����SO2�����ǽ��л������ã����ǿ�������Դ����Ŀǰ���������ԴΪ����Դ����Ϊ��ԭ����Դ�ḻ��ȼ�յIJ�������ˮ���Ȳ���Ⱦ�������ֿ���ѭ�����á�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
