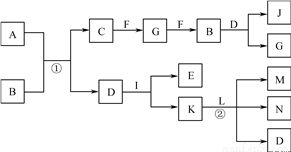
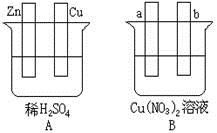
��Ŀ����
��֪���������[(NH4)2SO4��FeSO4��6H2O] ���׳�Ī���Σ�������ˮ����100�桫110��ʱ�ֽ⡣Ϊ̽���仯ѧ���ʣ��ס�����ͬѧ���������ʵ�顣
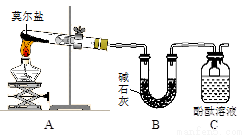
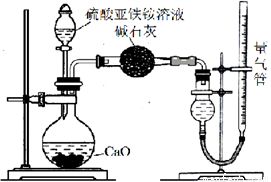
��̽��Ī���ξ������ʱ�ķֽ���
��ͬѧ�������ͼ��ʾ��װ�ý���ʵ�飬װ��C�пɹ۲쵽��������____________________���ɴ˿�֪�ֽ��������_______________��
��ͬѧ��ΪĪ���ξ���ֽ�IJ����л����ܺ���SO3(g)��SO2(g)��N2(g)��Ϊ��֤����Ĵ��ڣ�������װ�ý���ʵ�顣
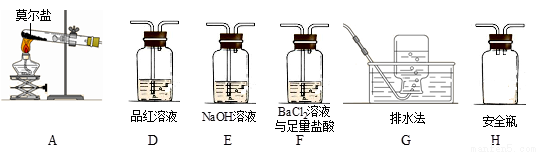
����ͬѧ��ʵ���У�װ���������ӵĺ���˳��Ϊ��A��H����____������____������____����G��
��֤������SO3��ʵ��������______________����ȫƿH��������____��
��Ϊ�ⶨ��������林��ȣ���ȡm gĪ������Ʒ�����500 mL��Һ���ס�����λͬѧ�������������ʵ�鷽����������ȡ25.00 mL��Ʒ��Һ��0.1000 mol��L��1������K2Cr2O7 ��Һ�����ν��еζ����ҷ�������ͨ��NH4+�ⶨ��ʵ�����װ������ͼ��ʾ��ȡ25.00 mL��Ʒ��Һ���и�ʵ�顣
��ش�
��1�������е����ӷ���ʽΪ��________________________��
��2���ҷ�����������������Լ���________
a��ˮ b������NaHCO3��Һ c��CCl4
��3���ҷ������ռ������岢�ָ������£�����ǰӦ���еIJ�����________________��
��4�������NH3ΪV L(������Ϊ��״����)������������林���Ϊ_____���г�����ʽ����
 ������ϵ�д�
������ϵ�д�I. Ϊ�Ƚ�Cl2��Fe3+��SO2�������ԣ�����ͼ��ʾװ�ý���ʵ�飬��������£�

��.���ɼ�K1��K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
��.����a���μ�һ������Ũ���ᣬ��A���ȡ�
��.��B�е���Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2��
��.����b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
��.���ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3��
��.�����Թ�D���ظ����̢ܣ�����B��Һ�е����ӡ�
��1�����н������ҺΪ__________��
��2����A�������������36.5%�ܶ�Ϊ1.2g/mL����100mLʱ���䷴Ӧת�Ƶĵ�����ĿΪ______��
��3�����̢��м���B��Һ���Ƿ�����������ӵIJ�����___________��
��4���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬���ǵļ����һ���ܹ�֤�������Ե���____ͬѧ����������˳��Ϊ____________��
���̢� B��Һ�к��е����� | ���̢� B��Һ �к��е����� | |
�� | ��Fe3+��Fe2+ | ��SO42�� |
�� | ����Fe3+����Fe2+ | ��SO42�� |
�� | ��Fe3+��Fe2+ | ��Fe2+ |
II. NaNO2����Ҫ�ķ�������+3�۵ĵ�����������Ӧ�������Ի����в��ȶ�����5mol/Lˮ��ҺpHΪ9��ij��ѧ��ȤС���������ͼ��ʾװ���Ʊ��������ơ�������Ǣٹرյ��ɼУ���A�з�Һ©���������μ�һ����Ũ���ᣬ���ȣ���һ��ʱ���ֹͣ���ȡ��ش��������⣺
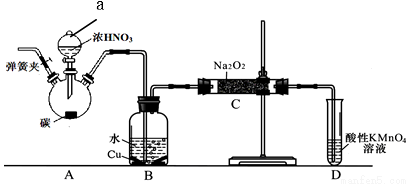
��1��B�й۲����Ҫ������__________��Dװ�õ�������____________��
��2������C�в������������ƵIJ�����_______����Ӧ��Ӧ����ʽΪ___________��
��3��������C�в����������ƺ������١�a. ��ͬѧ��ΪC�в��ﲻ�����������ƣ������������ʡ�Ϊ�ų����ţ�����B��Cװ�ü�����װ��E��E��ʢ�ŵ��Լ���_______��д���ƣ���b. ��ͬѧ��Ϊ���������������⣬�������������뷴Ӧ���²�Ʒ������������ʵ�������ǰӦ����һ���������ò�����___��
��4���������������HNO2��Ka��ֵΪ______________��
��ҵ����ȡCuCl2�������������£�

�����±����ݣ��ش��������⣺
�� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��