��Ŀ����
(11��)�±������������г��������ʣ������г������ǵģ���Ҫ���ɷ֡�
��1������Ա��Т١��ߵ���Ҫ�ɷֽ��з��ࣨ���ţ���
�����ε��� �����ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��2��д�������ڵ�ˮ��Һ��߷�Ӧ�Ļ�ѧ����ʽ ��
����������߷�Ӧ�����ӷ���ʽ ��
��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��
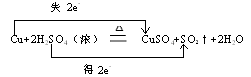
Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
��������ת�Ƶķ������Ŀ��������ת��0.1molʱ�����Ļ�ԭ��������Ϊ g��
| ��� | �� | �� | �� | �� | �� | �� | �� |
| ���� | �ƾ� | ���� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
| ��Ҫ �ɷ� | CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
�����ε��� �����ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��2��д�������ڵ�ˮ��Һ��߷�Ӧ�Ļ�ѧ����ʽ ��
����������߷�Ӧ�����ӷ���ʽ ��
��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��
Cu+2H2SO4��Ũ��
 CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O��������ת�Ƶķ������Ŀ��������ת��0.1molʱ�����Ļ�ԭ��������Ϊ g��
�� �ܢ�(1��)���ڢۢܢ�(1��);�٢�(1��)
�� 2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O (2��); H++CO32-=HCO3- (2��)
�� �������ת�Ƶķ������Ŀ��1�֣�
3.2g(2��)
�� 2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O (2��); H++CO32-=HCO3- (2��)
�� �������ת�Ƶķ������Ŀ��1�֣�

3.2g(2��)
�����������1�������ε���ʳ�Ρ��մ����ڵ���ʵ��д��ᡢ��ʳ�Ρ��մ����ڷǵ���ʵ��оƾ�������������
��2��������̼���Ʒ�Ӧ�ķ���ʽΪ��2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O������������̼���Ʒ�Ӧ�����ӷ���ʽΪ��H++CO32-=HCO3-����дʱ��Ӧ�ر�ע��������Զ��ٶԷ�Ӧ��Ӱ�졣
��3��������ԭ��Ӧ�е���ת������ı�ʾ����˫���ű�ʾ����ͼ��

���Ļ�ԭ����������
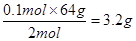
�����������ۺϿ����֪ʶ��϶࣬�����ǻ����Ե����ݣ����ڻ����⡣���������͵Ŀ��飬���ɿ���ѧ���Ի���֪ʶ�����գ��ֿ��Խ�϶�����ݡ��Ի���֪ʶ��������������˿����������ĵ÷������

��ϰ��ϵ�д�
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
�����Ŀ
 Si3N4 + CO
Si3N4 + CO